The toxicity of thiamine hydrochloride
Introduction
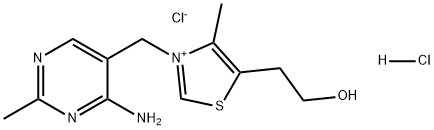
Thiamine hydrochloride is the hydrochloride of thiamine, also known as thiamine. It is the earliest water-soluble vitamin purified by people. The chemical name is 3-[(4-amino-2-methyl-5-pyrimidinyl) chloride. -Methyl]-5-(2-hydroxyethyl)-4-methylthiazole, chemical formula C12H17ClN4OS·HCl, is a form of vitamin B1. It has the function of maintaining normal glucose metabolism, and is often used clinically to prevent and treat beriberi caused by lack of VB1.
Properties
Its generic names are thiamine hydrochloride and vitamin B1. Its trade names are vitamin B hydrochloride, thiamine chloride hydrochloride, aneurine hydrochloride, and thiaminium-chloride. Its molecular weight is 337.25. It is colourless crystals or white crystalline powder with a characteristic somewhat meat-like odor and a bitter taste. It occurs in plants and in animal tissues, notably in rice husk, cereal grains, yeast, liver, eggs, milk, green leaves, roots and rice bran. Its melting range is 248° with decomposition. Its one gram dissolves in about 1 ml of water, 18 ml glycerol, 100 ml 95% alcohol, 315 ml abs. alcohol, more soluble in methanol soluble, in propylene glycol. It is practically insoluble in ether, benzene, hexane, chloroform.

Picture 1 Thiamine hydrochloride
The use of VB1
In view of the conservation of the environment combining with economic aspects, literature demands the application of metal ion free, environmentally safe, and convenient reagents in the multicomponent reactions. It is well known that thiamine hydrochloride (VB1) is a cheap and non-toxic reagent. The structure of VB1 contains a pyrimidine ring and a thiazole ring linked by a methylene bridge. The use of VB1 analogs as powerful catalysts for various organic transformations has been reported. In this Letter, we wish to report a facile, efficient, and practical method for the preparation of amidoalkyl naphthols in excellent yields using VB1 as a catalyst[1].
The crystal structure
The crystal structure of thiamine hydrochloride has been determined by three-dimensional Patterson superposition methods and refined by full-matrix least-squares computations to a final R factor of 8.0% for 3039 reflections with measurable intensities. The unit cell is monoclinic, space group P21/c, with a--6-99, b = 20.59, c = 12.73/~ and fl = 114.0 °. The locations of 17 out of 20 hydrogen atoms in the asymmetric unit have been determined from a difference-Fourier synthesis and ~n attempt has been made to refine the coordinates of these hydrogen atoms by least squares. The pyrimidine portion of the molecule is protonated at the ring nitrogen opposite the amino group. The planes of the thiazolium and pyrimidine rings are at a dihedral angle of 76 °, and are turned so as to bring the amino group closer to the hydrogen-bearing carbon of the thiazolium ring. The structure contains two weak but distinct C-H • • • C1- hydrogen bonds.
Toxicity
The above results are the same in every way as those observed when a solution containing 100 mg./cc. of thiamine hydrochloride and 0.35 per cent chlorobutanol was injected intravenously. The toxicity encountered upon injection of 100 mg./cc. of thiamine hydrochloride solutions is due to the thiamine content and not to the preservative. Symptoms of thiamine hydrochloride toxicity may be summarized as follows: (a) peripheral vaso[1]dilatation; (b) decreased respiration due to direct. action on the respiratory center in the medulla; (c) asphyxia1 convulsions due to anoxia resulting from decreased oxygenation of the blood; (d) death by paralysis of the respiratory center; and (e) cardiac arrhythmias, probably due to anoxia and not a direct action of thiamine hydrochloride on the cardiac muscle or the conducting system. Anaphylaxis plays no part in thiamine hydro[1]chloride toxicity as seen in rabbits. However, injec[1]tion of a sensitizing dose apparently increases the re[1]sistance of the animal to toxic injections of thiamine hydrochloride. The lethal dose of thiamine hydrochloride by intravenous injection into rabbits is approximately 126 mg./kg. After a sensitizing dose of 100 mg. of thiamine hydrochloride the lethal dose is approximately 238 mg./bg.
Application
A facile, efficient, and environmentally friendly procedure for the synthesis of 2,3-dihydroquinazolin-4(1H)-ones from isatoic anhydride, aldehyde, and ammonium acetate in the presence of thiamine hydrochloride (VB1) in EtOH is described. The protocol proves to be efficient and environmentally benign in terms of high yields, ease of recovery, and reusability of catalyst.
Reference
1 Wrenn K D, Murphy F, Slovis C M. A toxicity study of parenteral thiamine hydrochloride[J]. Annals of emergency medicine, 1989, 18(8): 867-870.
Related articles And Qustion
See also
Lastest Price from Thiamine hydrochloride manufacturers
US $0.00-0.00/kg2025-12-01
- CAS:
- 67-03-8
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 1000kg

US $1.00-4.00/KG2025-09-11
- CAS:
- 67-03-8
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 200000KG