The synthesis of 2-Thiouracil and its precautions
General description
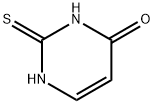
2-Thiouracil, a thiouracil derivative with the chemical formula C4H4N2OS, is a compound of interest primarily in the fields of medicine and biochemical research. It is structurally similar to uracil, a component of RNA, but with a sulfur atom replacing an oxygen atom in the uracil ring. This modification imparts unique biochemical properties to 2-Thiouracil, making it a valuable tool in various research and therapeutic contexts. However, the use of 2-Thiouracil also requires careful consideration of its potential effects and limitations.
Synthesis
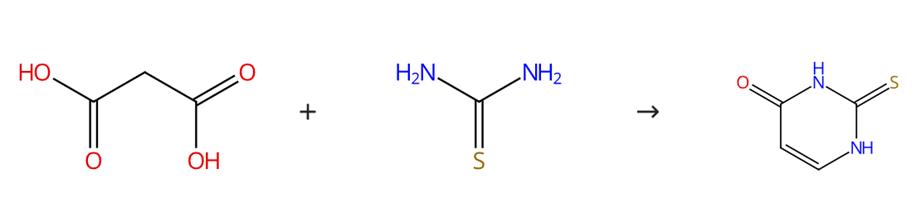
Fig. 1 The synthesis route of 2-Thiouracil
SchemeReflux a suspension of resin (500 mg) in triethyl orthoformate (50 equivalents, 5 mL) for 6 hours. Add urea (5 equivalents) to the reaction mixture. Reflux the mixture for 12 hours. Filter the resin. Wash the resin with 3 x 5 mL of EtOH and 3 x 5 mL of CH2Cl2. Cyclize the resin with an oil bath thermally at 240 °C for 20 minutes under N2atmosphere. Wash the mixture with EtOH/acetone completely in the sintered glass funnel. Combine the filtrates. Evaporate the filtrates. The synthesis route is shown in Fig. 1[1].
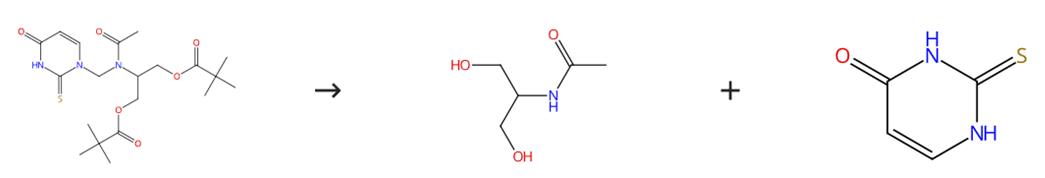
Fig. 2 The synthesis route of 2-Thiouracil
Scheme2 Keep a mixture of 1-[N-(1,3-dipivaloyloxyprop-2-yl)acetylaminomethyl]-5-methyl-1H,3H-pyrimidin-2,4-dione (1 mmol), concentrated ammonium hydroxide (20 cm3) and methanol (20 cm3) at 70°C (in a sealed tube) for 1 day. Evaporate the reaction mixture to dryness. Treat the residue with methanol (15 cm3). Filter the resulting suspension. Wash the solid with cold methanol (3 x 10 cm3). Dry the solid in the air. Combine the filtrate and the washings. Evaporate the filtrate to dryness. Isolate the product by column chromatography (chloroform-methanol, 95/5, v/v). The synthesis route is shown in Fig. 2[2].
Scheme3 Stir a mixture of N-[1,3-bis(pivaloyloxy)prop-2-yl]acetamide (3.0 g), sodium hydride (60% suspension in mineral oil, 1.0 g, 25.0 mmol) and dry dimethylformamide (DMF) (40 cm3) at room temperature for 1 hour. Cool the mixture in an ice bath. Add chloromethyl pivalate (6.89 g, 4.9 cm3) to the resulting suspension in one portion. After 4 days of stirring at room temperature, pour the mixture into ice water (120 cm3). Separate the phases. Extract the aqueous phase with ethyl acetate (4 x 30 cm3). Combine the extracts and the organic phase. Wash the extracts and the organic phase with brine. Dry the extracts and the organic phase. Distill off the low boiling solvent. Remove the residual DMF under vacuum (80°C, 0.05 mmHg). Purify the residue by column chromatography (dichloromethane)[3].
precautions
Firstly, the safety profile of 2-Thiouracil must be carefully considered. Like many pharmaceuticals and research chemicals, 2-Thiouracil has the potential for toxicity, particularly with long-term exposure. Studies have indicated that 2-Thiouracil can be hepatotoxic and may induce goitrogenic effects. Researchers and clinicians must adhere to safety guidelines for handling, storage, and disposal of 2-Thiouracil to minimize health risks. This includes the use of appropriate personal protective equipment (PPE), such as gloves and safety goggles, and ensuring that work with the compound is conducted in well-ventilated areas or fume hoods.
Secondly, the environmental impact of 2-Thiouracil warrants attention. As with any chemical compound, improper disposal of 2-Thiouracil can lead to environmental contamination, affecting water sources and ecosystems. Facilities using 2-Thiouracil should have strict waste management protocols in place to prevent environmental pollution, including specific measures for chemical waste treatment and disposal.
In addition to safety and environmental considerations, the legal and regulatory aspects surrounding the use of 2-Thiouracil must be observed. Depending on the jurisdiction, there may be regulations governing the acquisition, use, and disposal of 2-Thiouracil, particularly in a research or clinical setting. It is incumbent upon users to familiarize themselves with these regulations and ensure compliance to avoid legal issues and ensure the ethical conduct of research.
In conclusion, while 2-Thiouracil remains a valuable compound for research and previously in medical treatment, its use comes with a set of important precautions. Awareness of its pharmacological effects, adherence to safety protocols, consideration for environmental impact, and compliance with legal regulations are essential for the responsible use of 2-Thiouracil. By taking these considerations into account, researchers and clinicians can mitigate risks and contribute to the safe and effective use of this compound in their work.
References
[1]Huang, Xian; et al.Solid-Phase Synthesis of 4(1H)-Quinolone and Pyrimidine Derivatives Based on a New Scaffold-Polymer-Bound Cyclic Malonic Acid Ester.Journal of Organic Chemistry (2002), 67(19), 6731-6737.
[2] Koszytkowska-Stawinska, Mariola.Studies on the Synthesis of N'-Acetyl Aza-Analogs of Ganciclovir-Unexpected Liability of N'-(2-Hydroxyethyl)-aza-nucleosides Under Basic Conditions.
Nucleosides, Nucleotides & Nucleic Acids (2010), 29(10), 768-785.
[3]Koszytkowska-Stawinska, Mariola.Studies on the Synthesis of N'-Acetyl Aza-Analogs of Ganciclovir-Unexpected Liability of N'-(2-Hydroxyethyl)-aza-nucleosides Under Basic Conditions. Nucleosides, Nucleotides & Nucleic Acids (2010), 29(10), 768-785.
Related articles And Qustion
See also
Lastest Price from 2-Thiouracil manufacturers

US $0.00/kg2025-09-01
- CAS:
- 141-90-2
- Min. Order:
- 1kg
- Purity:
- 99%min
- Supply Ability:
- 20tons
US $0.00-0.00/Kg2025-04-21
- CAS:
- 141-90-2
- Min. Order:
- 1Kg
- Purity:
- 98%
- Supply Ability:
- 20Ton