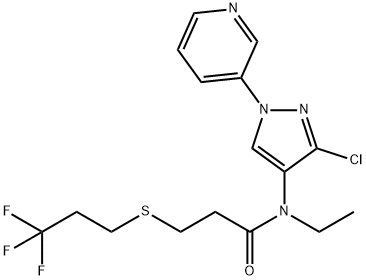
The synthesis method of tyclopyrazoflor
Description
Tyclopyrazoflor (GF-3242) is a pyridinyl–pyrazole insecticide with excellent activity against sap-feeding insects (especially against aphids), first disclosed by Dow (now Corteva) in 2013 and announced by Corteva Agriscience as a new insecticide in 2017[1]. The compound shows good control of M. persicae and sweet potato whitefly crawler and is the first representative of a new chemical class. The mode of action of tyclopyrazoflor is still unknown[2].
Synthesis method
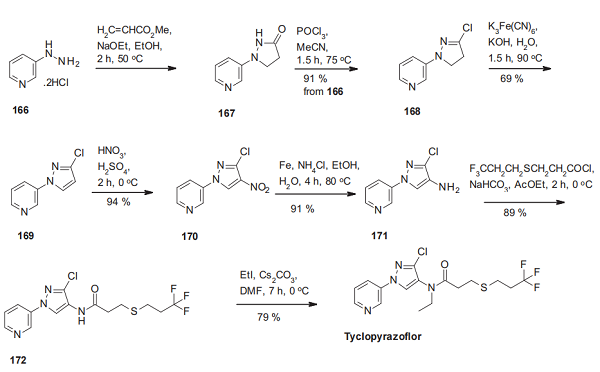
The synthesis of tyclopyrazoflor starts with a [3 + 2] cyclization of 3-hydrazinopyridine hydrochloride (166) and methyl acrylate. Chlorination of the resulting pyrazolidinone 167 leads to the pyrazoline 168, which is oxidized to the disubstituted pyrazole derivative 169. Regioselective nitration delivers the nitropyrazole 170, which is then reduced to the corresponding aminopyrazole derivative 171. Its acylation with 3-((333-trifluoropropyl)thio)propanoyl chloride affords the secondary amide 172, which is then converted into tyclopyrazoflor by alkylation[3].
References
[1] Meijun Chen, Xusheng Shao*, Zhong Li and Peter Maienfisch*. “Bioisosteric-Replacement-Driven Lead Optimization of Tyclopyrazoflor.” Journal of Agricultural and Food Chemistry 70 36 (2022): 11123–11137.
[2] Kaitlyn C. Gray*. “Development of a Scalable Process for the Insecticidal Candidate Tyclopyrazoflor. Part 3. A Scalable Synthesis of Methyl 3-((3,3,3-Trifluoropropyl)thio)propanoate via Thiol–Ene Chemistry.” Organic Process Research & Development 23 10 (2019): 2142–2147.
[3] Stephane Jeanmart . “Synthetic approaches to the 2015–2018 new agrochemicals.” Bioorganic & Medicinal Chemistry 39 (2021): Article 116162.