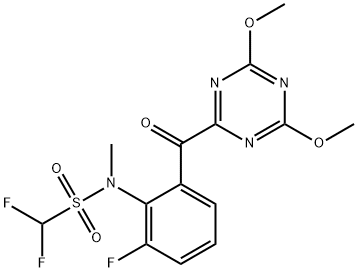
Synthetic route to the herbicide Triafamone
Synthesis of Triafamone
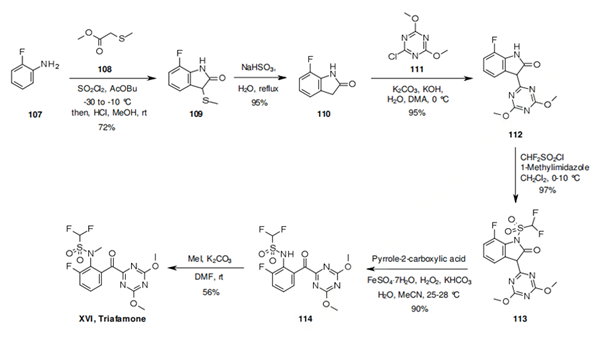
The
synthesis of triafamone begins by the reaction of aniline 107 and methyl 2-
methylthioacetate (108) in the presence of sulfuryl chloride, which yields indolone 109. Reductive cleavage of the methylthiol group, followed by SNAr reaction of intermediate 110
with triazine 111 under basic conditions delivers indolone 112. The sulfonamide 113 is easily
obtained by treating 112 with difluorosulfonyl chloride in the presence of 1-methylimidazole. The benzophenone motif of triafamone is finally revealed by the oxidation of the indolone 113 using an iron sulfate and hydrogen peroxide mixture, and its synthesis completed after
methylation of the advanced intermediate 114.
Introduction of Triafamone
Triafamone (approved ISO common name) is a new sulfonanilide herbicide discovered and developed by Bayer CropScience AG under the code number AE 1887196. Its mode of action is inhibition of the enzyme acetolactate synthase (ALS). Field trials since 2007 have shown that triafamone can be effectively used in direct seeded or transplanted rice from seeding or transplanting to late post-emergence at rates of 20 to 50 g a.i./ha using spray or granular formulations. Target weeds are important grasses such as Echinochloa crus-galli, Echinochloa colonum, Echinochloa oryzicola, Paspalum distichum, Isachne globosa, and sedges (including ALS resistant strains).