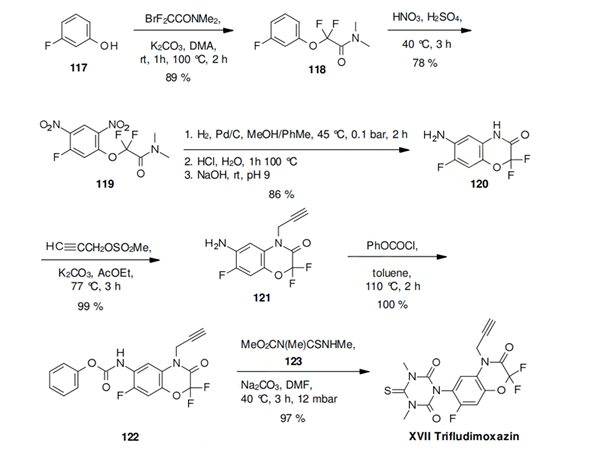
Synthetic route to the herbicide Trifludimoxazin
Synthesis of Trifludimoxazin
The synthesis of trifludimoxazin starts with the alkylation of 3-fluorophenol (117) with bromodifluorodimethylacetamid. The resulting aryloxyacetamide 118, is nitrated to the tetrasubstitited phenyl derivative 119. After reduction of the nitro groups, the benzoxazinone derivative 120 is formed, bearing three different fluoro substituents, two of which are located in the oxazinone ring and the third one in the phenyl ring. Propargylation of the ring nitrogen, with subsequent protection of the amino function as a phenyl carbamate yields the intermediate 122. This can be directly cyclized to trifludimoxazin with N-methoxycarbonyl-N,N-dimethylthiourea (123) under basic conditions.
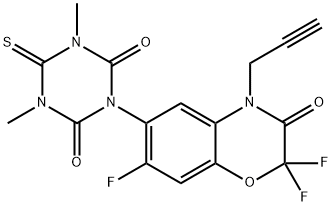
Introduction of Trifludimoxazin
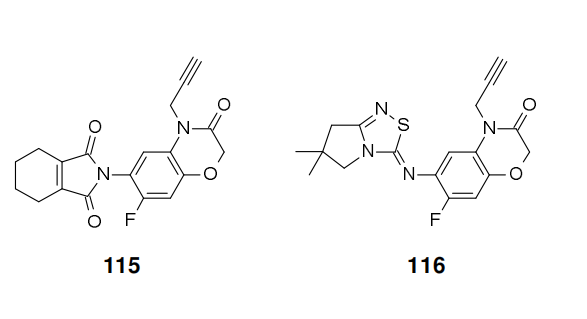
The triazinone herbicide trifludimoxazin (XVII) was reported by BASF in 2014. After flumioxazin (115) and thidiazimin (116) , it will be the third benzoxazinone derivative amongst the group of protoporphyrinogen oxidase (PPO) inhibitors. However, in contrast to this new compound, the two aforementioned established market products neither contain trifludimoxazin's dimethylated thiocyanuric acid moiety nor bear the gem-difluoro substitution within its oxazinone ring.