Synthetic route to the herbicide Halauxifen-methyl
Synthesis of Halauxifen-methyl
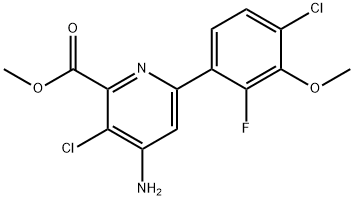
Two types of synthetic routes have been reported in process patents from Dow AgroSciences: One of these involves the de novo synthesis of the pyridine ring, while the other utilizes a cross-coupling reaction to generate the biaryl system. The later was chosen to be reported in this review as the scale described in process patents, and the number of process patents published suggests this is the favored route. Following the cross coupling strategy, halauxifen-methyl is prepared from the 2-chloro-6-fluoroanisole 124 by metallation, and borylation to yield boronic acid 125. Suzuki reaction of 125 with 126 yields 127 which is subsequently deprotected, to the title product. The two last steps are reported to give an overall yield of 90%.
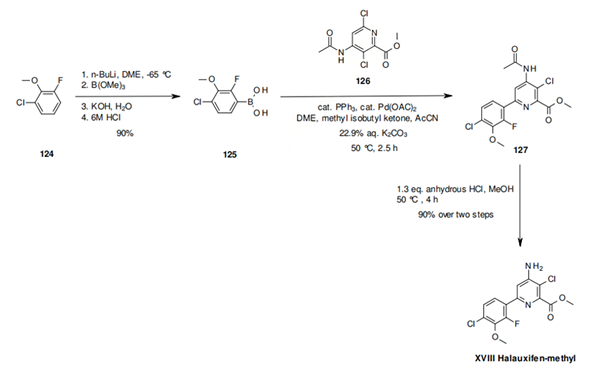
Introduction of Halauxifen-methyl
Halauxifen-methyl is a new herbicide launched as Arylex™ by Dow AgroSciences. Halauxifen-methyl is the first member of the new phenyl aminopyralid class of synthetic auxin herbicides. The compound is cleaved in planta to the carboxylate which is the active ingredient. Halauxifen provides a step change in the control of broadleaf weeds as it is active at much lower rates than classical auxin herbicides.