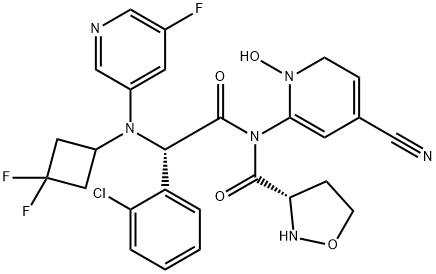
The synthesis method of Ivosidenib
Description
Ivosidenib, a first-in-class, oral small molecule, potent and selective inhibitor of mutant isocitrate dehydrogenase 1 (mIDH1), is approved in the EU and USA for the treatment of adults with pretreated, advanced, mIDH1 cholangiocarcinoma (CCA). The USFDA approved it in July 2018 for treating adult patients with relapsed or refractory acute myeloid leukemia (AML) with a susceptible IDH1 mutation, as detected by a US FDA-approved test[1].
IDH1 mutations have been identified in various hematologic and solid tumors, conferring a gain of function in cancer cells and resulting in increased levels of the oncometabolite 2-hydroxyglutarate (2-HG). 2-HG plays a critical contributory role in leukemia tumorigenesis. Ivosidenib has been shown to reduce 2-HG levels, which are key contributors to leukemia tumorigenesis and translated to durable remissions during clinical trials.
Synthetic method
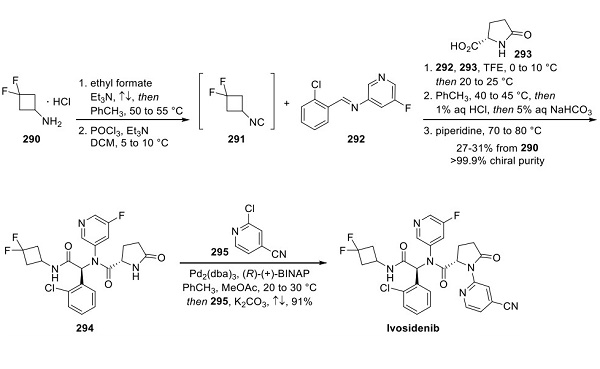
Figure 1. Synthesis of Ivosidenib
While patents from Agios have described only a general procedure for the synthesis of ivosidenib, with no isolation details or yields, Teva Pharmaceuticals has published a process that provides the API in high chiral purity without column chromatography(shown in Figure 1)[2]. Amine 290 was converted to the corresponding formamide by treatment with ethyl formate in the presence of triethylamine. Following partial evaporation of the solvent, dehydration of the formamide (POCl3, Et3N) afforded isonitrile 291, which was dissolved in trifluoroethanol and carried forward crude into the key Ugi reaction with imine 292 (performed by condensation of aniline 296 with aldehyde 297, as shown figure 2) and acid 293. While the Ugi reaction provided the product as a mixture of diastereomers, a workup and crystallization procedure was developed to isolate the desired diastereomer 294. Acid/ base extraction and treatment with piperidine allowed for the isolation of pure 294 in >99.9% chiral purity and 27−31% yield from amine 290. Buchwald−Hartwig coupling with 2- chloroisonicotinonitrile (295), followed by crystallization from EtOAc and n-heptane, gave ivosidenib a 91% yield.
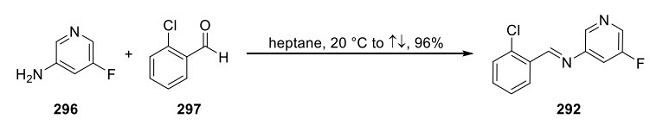
Figure 2. Synthesis of Ivosidenib Imine 292
References
[1] Frampton, James E. “Ivosidenib: A Review in Advanced Cholangiocarcinoma.” Targeted Oncology (2023): 973–980.
[2] Andrew C. Flick. “Synthetic Approaches to New Drugs Approved during 2018.” Journal of Medicinal Chemistry 63 19 (2020): 10652–10704.
You may like
See also
Lastest Price from Ivosidenib manufacturers
US $0.00-0.00/KG2025-12-11
- CAS:
- 1448347-49-6
- Min. Order:
- 1KG
- Purity:
- 98
- Supply Ability:
- 10000KGS