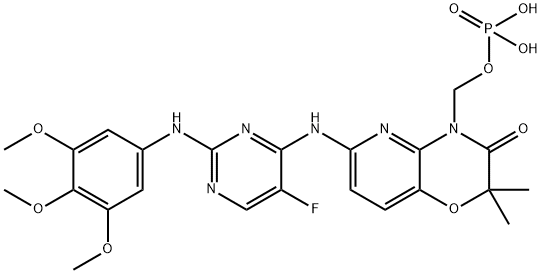
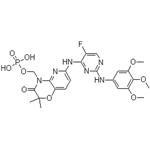
The synthesis method of Fostamatinib
Description
Fostamatinib was approved by the USFDA in 2018 for treating thrombocytopenia in adults with chronic immune thrombocytopenia (ITP) who have not demonstrated sufficient response to prior treatment. Due to the low aqueous solubility of the drug's active form (R406), fostamatinib is marketed as a methylene phosphonate prodrug, which undergoes conversion to the active metabolite of the drug in the gut. In clinical trials, ITP patients (even those who did not previously respond to splenectomy, rituximab, and/or thrombopoietic agent treatments) demonstrated statistically significant responses when treated with fostamatinib. At present, studies involving the drug as a treatment for other indications, such as IgA nephropathy and hemolytic anemia, are ongoing.
Biological activity
Fostamatinib is an inhibitor of spleen tyrosine kinase (SYK), a key regulator of signal transduction pathways involved in various autoimmune diseases. Fostamatinib inhibits Fcγ receptor (FcγR)-mediated signal transduction, preventing cytoskeletal rearrangement and decreasing platelet destruction.
Synthetic route
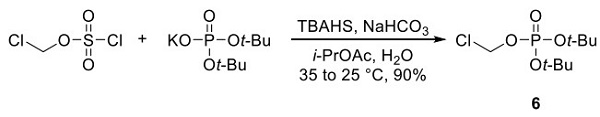
A large-scale synthetic route to fostamatinib has been reported by Rigel Pharmaceuticals[1]. Phosphate 6 is one of the raw materials. As shown above, it was prepared in a single step from chloromethyl sulfurochloridate and potassium di-tert-butyl phosphate.
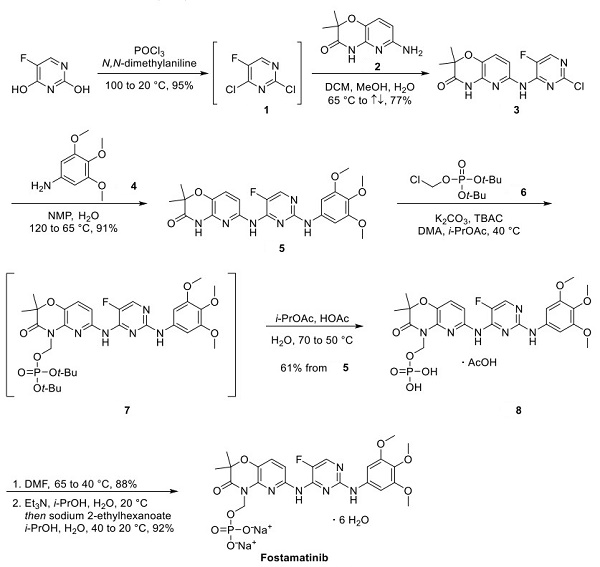
After that, the Chlorination of pyrimidine with POCl3 required a high temperature. Upon cooling, monosubstitution with aminopyridine 2 occurred regioselectively to provide pyrimidine 3 in 77% yield. They substituted chloropyrimidine 3 with amine 4, which required heating in aqueous NMP to furnish intermediate 5 in 91% yield. Impressively, studies by AstraZeneca showed that conditions could provide compound 5 at over 500 kg in single-batch reactions. Installation of the phosphate side chain was demonstrated by alkylation of 5 with tertbutyl phosphate 6 under primary conditions. Extraction with i-PrOAc led to a solution of intermediate 7, which was not isolated but heated with aqueous acetic acid, facilitating tertbutyl cleavage and precipitation of phosphonic acid intermediate 8 as an acetic acid solvate. Conversion to the DMF solvate enabled isolation of clean product in 88% yield after washing with MTBE. The final step of the synthesis was performed on a 1 kg scale. It was accomplished by reacting the DMF solvate of 8 with triethylamine in i-PrOH at room temperature, filtration, and subjection of the filtrate solution to sodium 2-ethylhexanoate in water and i-PrOH. Fostamatinib hexahydrate was isolated in 92% yield following precipitation from the reaction mixture.
References
[1] Andrew C. Flick. “Synthetic Approaches to New Drugs Approved during 2018.” Journal of Medicinal Chemistry 63 19 (2020): 10652–10704.
See also
Lastest Price from Fostamatinib manufacturers

US $0.00/g2025-04-21
- CAS:
- 901119-35-5
- Min. Order:
- 1g
- Purity:
- 98%min
- Supply Ability:
- 1000g
US $0.00-0.00/g2025-04-20
- CAS:
- 901119-35-5
- Min. Order:
- 10g
- Purity:
- 99% HPLC
- Supply Ability:
- 1000kg