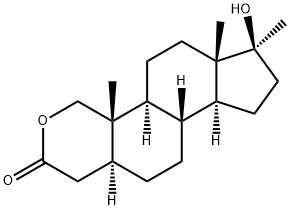
The Synthesis and Storage Methods of Oxandrolone
General description
Oxandrolone, a synthetic anabolic steroid, was first introduced in the United States in 1964. It is derived from dihydrotestosterone (DHT) and is known for its relatively mild androgenic properties compared to other anabolic steroids. Oxandrolone is widely used for promoting weight gain in various situations, such as after extensive surgery, chronic infections, or severe trauma. Additionally, it has been prescribed to counter the long-term catabolic state induced by corticosteroid therapy and for the relief of bone pain associated with osteoporosis.
Synthesis
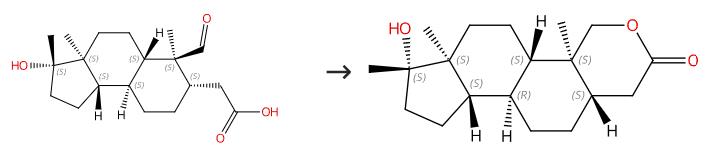
Fig. 1 The synthesis route of Oxandrolone
Scheme 1: Charge 1.10 kg (3.42 mol) of recrystallized 17-β-hydroxy-17-α-methyl-1-oxo-1,2-seco-A-nor-5-α-androstan-2-oic acid, 8.4 L of absolute ethanol, and 8.4 L of distilled water into a 50-L four-neck round-bottom flask equipped with a stir assembly, thermocouple, nitrogen inlet adapter, and 2-L addition funnel. Cool the slurry to an internal temperature of 8 °C. Add a 25% solution of NaOH (198 mL 50% NaOH, 198 mL water) to the reaction mixture over 16 min at an internal temperature of 8-9 °C. Add 188 g (5.0 mol) of NaBH4 in four equal portions to the resulting yellow solution of the carboxylate over 1.5 h at an internal temperature of 5-7 °C. Stir the solution for an additional 1 h when the addition is completed. Monitor the reaction completion by TLC analysis. Add a 6.0 M solution of HCl (0.9 L of 37% HCl, 0.9 L of water) to the solution containing the sodium salt of the seco-acid carefully over 4 h at an internal temperature of 1-9 °C. Stir the resulting thick slurry at 1-3 °C for 4.75 h. Monitor the reaction to be complete by TLC analysis. The slurry thins and becomes easier to stir as the seco-acid cyclizes. Filter the slurry. Wash the slurry with a 0-10 °C solution of 1/1 EtOH/water (664 mL of each) 4.2 L of water and finally 3.2 L of heptane. Dry the solids via high vacuum at 40-50 °C to a constant weight to obtain oxandrolone (17-β-hydroxy-17-α-methyl-2-oxa-5-α-androstane-3-one). The synthesis route is shown in Fig. 1 [1].
Scheme2:Add 25.0 g (82.2 mmol) of methylandrostanolone, 100 mL of absolute ethanol, and 25 mL of distilled water to a nitrogen-purged 1 L four-neck round-bottom flask equipped with a mechanical stirrer, thermocouple, addition funnel, and nitrogen inlet. Add 36.0 g (107 mmol) of pyridinium tribromide in 200 mL absolute ethanol to the slurry over 2 h at 24 to 27 °C. The orange color of the PyHBr3;persists through the addition. Thin the orange slurry to a fine orange suspension and eventually an off-white slurry forms. Stir the reaction mixture for an additional 3.25 h. Determine the reaction completion by TLC analysis. Add 6.0 g (24 mmol) of sodium thiosulfate pentahydrate in 100 mL of water to the off-white slurry over 10 min (pH ca. 1). Stir the snow-white slurry for 30 min at room temperature. Add 22.0 g (208 mmol) of sodium carbonate in 200 mL of water to the slurry over 5 min (pH 7-8). Stir the resulting slurry for 15 min. Filter the reaction mixture. Wash the white cake successively with 100 mL of a cold mixture of water/ethanol (0-5 °C, 2/1 (v/v)), 3 times with 125 mL of water, and once with ca. 100 mL of heptane. Dry the white solid at 50-60 °C in a high vacuum oven for 19 h to obtain 2-α-bromo-17-α-methyl-5-α-androstan- 17-β-ol-3-one[2].
Scheme3:Dissolve 17-β-hydroxy-17-α-methyl-5-α-androst- 1-ene-3-one (250 g, 0.828 mol) in 2.5 L of MeOH.Cool the resulting solution to -30 to -40 °C.Bubble ozone into the solution through a sparge tube. Monitor the reaction completion by TLC analysis after 6 h. Allow the solution to warm to -10 °C. Add an aqueous solution of NaOH (109 mL of concentrated NaOH in 3.13 L of H2O) dropwise to the reaction mixture over 2 h. An 8 °C exotherm is observed during the addition. Allow the reaction mixture to warm to ambient temperature. Remove methanol under reduced pressure (2.8 L removed). Wash the aqueous solution of the sodium salt with MTBE (2 x 1.0 L). Acidify the reaction mixture to a pH of 4 using 6.0 M HCl.Stir the reaction mixture for ca. 1 h. Filter the slurry. Wash the slurry with water (2 x 300 mL) and 200 mL of n-heptane. Dry the product to constant weight via high vacuum at 40-60 °C to obtain 17-β-hydroxy-17-α-methyl-1-oxo-1,2- seco-A-nor-5-α-androstan-2-oic acid[2].
Storage methods
The stability and potency of oxandrolone, like many pharmaceutical compounds, can be affected by its storage conditions. To maintain its efficacy, oxandrolone should be stored in a controlled environment. The recommended storage conditions typically include a cool, dry place away from direct sunlight. The temperature should be maintained within a specific range, usually at or below room temperature, approximately 20 to 25 degrees Celsius (68 to 77 degrees Fahrenheit). Humidity can also affect the drug's stability, hence, it is advisable to keep it in an area with low humidity. The original packaging often provides adequate protection against moisture and light, which are critical factors that can lead to the degradation of the compound.
References
[1] Cedarburg Pharmaceuticals, LLC. "Process for preparing oxandrolone from 17β-hydroxy-17α-methyl-5α-androstan-3-one." World Intellectual Property Organization, WO2002100881 A1, December 19, 2002.
[2] Cabaj, John E., et al. "Development of a Commercial Process to Produce Oxandrolone." Organic Process Research & Development 11, no. 3 (2007): 378-388.
Related articles And Qustion
See also
Lastest Price from Oxandrolone manufacturers

US $2400.00-2228.00/kg2024-10-23
- CAS:
- 53-39-4
- Min. Order:
- 1kg
- Purity:
- 99.9%
- Supply Ability:
- 500kg

US $35.00/Box2024-10-18
- CAS:
- 53-39-4
- Min. Order:
- 1Box
- Purity:
- 99.99%
- Supply Ability:
- 10000000000