The special properties and applications of 2-furoic acid
Introduction
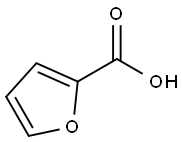
2-Furoic acid (Figure 1), also known as Pyromucic acid, is a raw material used in the synthesis of products such as tetrahydrofuran, furfurylamide, and furfurfuryl esters. 2-Furoic acid can be used in the plastic industry to produce plasticizers, thermosetting resins, etc; It can be used as a mold inhibitor and preservative in the food industry. The modified alkyd resin with 2-furoic acid as the main raw material has strong adhesion, fast drying, and good comprehensive performance of the paint film. 2-Furoic acid can also be used as an intermediate for the synthesis of a new anti-cancer drug, making it widely applicable. China is a large agricultural country with abundant resources of agricultural and sideline products. Using agricultural waste such as rice bran, corn stalks, corn cobs, sugarcane bagasse, wheat stalks, etc., once treated with sulfuric acid, 2-furanaldehyde is first obtained, and then 2-furoic acid is produced. In this way, agricultural and sideline products have been comprehensively utilized.

Thermal degradation of 2-furoic acid
The decarboxylation of 2-furoic acid (2-FA) was envisaged as an alternative pathway leading to furan in food [1]. The objective of the current study is to further examine the possible participation of 2-furoic acid in the formation of furan in heat-treated foods with particular emphasis on degradation mechanisms involved along coffee roasting. Furfuryl alcohol and 2-furoic acids were measuredin a selection of roasted coffee products by isotope dilution liquid chromatography-high resolution mass spectrometry, and the data evidenced a strong correlation between the two compounds, suggesting an intimate mechanistic relationship between them. The possible oxidation of furfuryl alcohol to furfural and 2-furoic acid in heated food is raised with particular emphasis on coffee roasting.
The thermal degradation of 2-furoic acid was monitored initially by running incubations of each standard individually for 20 min at different temperatures from 90 to 190 ℃. Evidence for the decarboxylation of 2-furoic acid was obtained by the temperature-dependent increase in furan observed over the range of temperature under investigation. No furan was detected at temperatures below130 ℃, whilst a significant increase from 77.1 μmol per mol of 2-furoic acid found at 130 ℃ to 977.4 μmol per mol of 2-furoic acid at 180 ℃ was observed.At 190 ℃, the conversion rate of 2-furoic acid to furan is ca. 0.1%. The thermal energy required for the cleavage of the C-C bond between the furan ring and the carboxylic function (as from 150 to 160 °C) is consistent with previous observations carried out with pipecolic acid betaine orindomethacin that typically decarboxylate at a similar temperature level, i.e. 160–170 °C. To a far lower extent, a small amount of 2-MeF was detected, with a conversion rate of 2-furoic acid to 2-MeF of only 0.0003%at 190 °C. In a second set of experiments, the formation of furan and 2-MeF from thermal degradation of 2-furoic acid was studied at constant temperature (190 °C) in a time-dependent manner with incubations up to 30 min. The formation of furan by decarboxylation of 2-furoic acid commenced after 5-min incubation time (mean conversion value at 0.19 mmol furan per mol 2-furoic acid) and kept increasing up to rate of 1.33 mmol furan per mol 2-furoic acid with a 30-min incubation time(conversion rate at ca. 0.15%).These findings are relevant for better understanding the formation of furan and alkylfurans in food, and ultimately opening avenues for mitigation.[2]
Nematicidal activity of 2-furoic acid
The test concentrations utilized in the nematicidal activity assay for M. incognita were 400, 200, 100, 50, and 25 µg/mL, and the results showed that 2-furoic acid had excellent nematicidal activity. At 48 h, the mortality rate of M. incognita was greater than 90% at 2-furoic acid concentrations of 400 and 200 µg/mL. At a concentration of 50 µg/mL, the lethality rate of M. incognita reached 50% at 48 h. The LD50 value of 2-furoic acid for M. incognita at 48 h was 55.05 µg/mL. They also evaluated 2-furoic acid’s lethality on P. redivivus and C. elegans; it has also shown great nematicidal ability, and after 48 h, its respective LD50 values were 3.33 and 147.63 µg/mL. The excellent nematicidal ability of 2-furoic acid and its general insecticidal effect indicate its potential in the development of biological control agents. 2-Furoic acid is a heterocyclic carboxylic acid that has been widely used in the pharmaceutical, agrochemical, flavor, and fragrance industries. In the industry, furfural is obtained by the depolymerization of xylose in hemicellulose, and xylose is produced after acid-catalyzed dehydration. Furfural is then catalyzed by aldehyde dehydrogenase to produce 2-furoic acid. Furthermore, the pot experiment showed that the number of galls of tomato root was significantly reduced in the experimental group treated with 2-furoic acid. The considerable increase in the 2-furoic acid content during the infection process and its virulent nematicidal activity revealed an essential synergistic effect during the process of nematode-trapping fungal infection.[3]
2-Furoic acid-assisted pretreatment for sugarcane bagasse biorefnery
In this work, 2-furoic acid, a novel recyclable organic acid as catalyst, was employed to pretreat sugarcane bagasse to recover the xylooligosaccharides fraction from hemicellulose and boost the subsequent cellulose saccharifcation. Results: The FA-assisted hydrolysis of sugarcane bagasse using 3% 2-furoic acid at 170 °C for 15 min resulted in the highest xylooligosaccharides yield of 45.6%; subsequently, 83.1 g/L of glucose was harvested by a fed-batch operation with a solid loading of 15%. Overall, a total of 120 g of xylooligosaccharides and 335 g glucose could be collected from 1000 g sugarcane bagasse starting from the 2-furoic acid pretreatment. Furthermore, 2-furoic acid can be easily recovered by cooling crystallization.Conclusion: This work put forward a novel 2-furoic acid pretreatment method to convert sugarcane bagasse into xylooligosaccharides and glucose, which provides a strategy that the sugar and nutraceutical industries can be used to reduce the production cost. The developed process showed that the yields of xylooligosaccharides and byproducts were controllable by shortening the reaction time; meanwhile, the recyclability of furoic acid also can potentially reduce the pretreatment cost and potentially replace the traditional mineral acids pretreatment.[4]
References
[1] Varelis P, & Hucker B. Thermal decarboxylation of 2-furoic acid and its implication for the formation of furan in foods. Food Chem. 2011;126:1512–1513.
[2] Delatour T, Huertas-Pérez JF, Dubois M, et al. Thermal degradation of 2-furoic acid and furfuryl alcohol as pathways in the formation of furan and 2-methylfuran in food. Food Chem. 2020;303:125406. doi:10.1016/j.foodchem.2019.125406
[3] Lei HM, Wang JT, Hu QY, et al. 2-Furoic acid associated with the infection of nematodes by Dactylellina haptotyla and its biocontrol potential on plant root-knot nematodes. Microbiol Spectr. Published online September 27, 2023. doi:10.1128/spectrum.01896-23
[4] Dai L, Huang T, Jiang K, Zhou X, Xu Y. A novel recyclable furoic acid-assisted pretreatment for sugarcane bagasse biorefinery in co-production of xylooligosaccharides and glucose. Biotechnol Biofuels. 2021;14(1):35. Published 2021 Feb 2. doi:10.1186/s13068-021-01884-3
You may like
See also
Lastest Price from 2-Furoic acid manufacturers

US $0.00-0.00/kg2025-05-09
- CAS:
- 88-14-2
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 1000kg

US $0.00-0.00/kg2025-04-21
- CAS:
- 88-14-2
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 100 mt