The role of N-fluorobisbenzenesulfonamide in chemical synthesis
Background
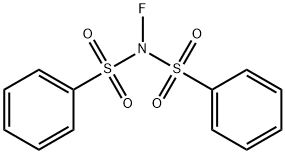
N-Fluorobenzenesulfonimide (NFSI) is a colorless crystalline powder with a melting point of 114–116 °C[1]. One of the most convenient routes to prepare NFSI in high yield is the fluorination with 10% v/v F2 in N2 of N-(phenylsulfonyl)benzenesulfonamide in MeCN at –40 °C. NFSI can also be prepared from the corresponding sodium salt. NFSI has been widely used as an electrophilic fluorinating agent most particularly in the field of asymmetric electrophilic fluorination. But NFSI is more than an electrophilic fluorinating agent: It is also a strong oxidant for organometallic intermediates to promote reductive elimination through high-oxidation-state transition metals, an amination reagent and even a phenylsulfonyl group transfer reagent.

Picture 1 NFSI powder
N-Fluorobisbenzenesulfonamide is a chemical substance with white crystals. It is easily soluble in organic solvents such as chloroform, dichloromethane and ethyl acetate. It has low solubility in ethanol and methanol, and is soluble in water and petroleum ether. Solubility is less. Stable to acid, decomposed by strong reducing agent, non-flammable.
NFSI as Fluorinating Reagent
Shibata and co-workers reported the enantioselective synthesis of 3′-fluorothalidomide through an electrophilic fluorination using a combination of cinchona alkaloid and NFSI. The appropriate choice of additives allowed enantiodivergent fluorination using a single alkaloid; indeed, TMEDA as ligand provided the S-enantiomer whereas bipy–Cu(acac)2 gave the R-enantiomer. Pure enantiomers were obtained after deprotection and oxidation. In the prospect of the synthesis of radiopharmaceuticals, such as 18F-fluorothalidomide, for positron emission tomography (PET) analysis, [18F]NFSI has been developed
Mazet, Alexakis and co-workers have developed a sequential iridium-catalyzed redox isomerization–organocatalytic fluorination. The intermediate aldehyde, which was the result of the redox isomerization of the starting primary allylic alcohol, was submitted to the electrophilic fluorination by means of NFSI through enamine catalysis with 20 mol% of a proline derivative. A quench by NaBH4 produced the fluoroalcohol that featured two stereogenic centers in an overall 49% yield with 99% ee for the main syn-diastereoisomer.
NFSI as Oxidizing Reagent
(C) NFSI has been used as a versatile oxidant in reactions that involve a PdII–PdIV catalytic cycle. Michael and co-workers described the palladium-catalyzed diamination, carboamination and alkoxyamination of unactivated alkenes using NFSI as an oxidant. The oxidative addition of NFSI allowed the formation of a PdIV intermediate; then reductive elimination led to the diamination product A. The PdIV intermediate could also react with nucleophilic arenes to form the carboamination product B through C–H activation followed by reductive elimination. When the reaction was performed in alcoholic solvents, the solvolysis of the PdIV intermediate led to the alkoxyamination 5-exo product C or through neighboring group participation to the 6-endo product D depending on solvent polarity.
NFSI as Amination Reagent
NFSI has also been used to realize the selective amination of various compounds. Zhang and co-workers described the amide-directed palladium-catalyzed selective aromatic C–H amination of anilides with NFSI in good yields. In addition, it was demonstrated that NFSI is the decisive reagent for the oxidative amination of C(sp3)–H bonds at benzylic positions under palladium or copper catalysis.
Liu and co-workers described an intermolecular Pd-catalyzed oxidative aminofluorination of vinyl arenes with NFSI in the presence of bathocuproine. NFSI functioned not only as an amination agent but also as a fluorination and an oxidizing reagent. The reaction affords vicinal fluoroamine products with very high regioselectivity.
NFSI as Phenylsulfonyl Transfer Reagent
The reaction of the purine C-8 carbanion of a protected 5′-noraristeromycin derivative with NFSI failed to give the expected 8-fluoroderivatives but gave the 8-phenylsulfonyl-5′-noraristeromycin instead. This phenylsulfonyl transfer proceeds via a single electron transfer.
Reference
1 Sun K, Li Y, Xiong T, et al. Palladium-catalyzed C− H aminations of anilides with N-fluorobenzenesulfonimide[J]. Journal of the American Chemical Society, 2011, 133(6): 1694-1697.
Related articles And Qustion
See also
Lastest Price from N-Fluorobenzenesulfonimide manufacturers
US $1.00/KG2025-04-21
- CAS:
- 133745-75-2
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt

US $30.00/kg2025-04-15
- CAS:
- 133745-75-2
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 5000kg/Month