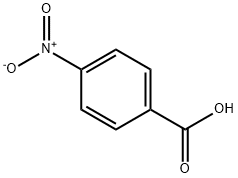
The preparation method of p-nitrobenzoic acid
Background
The appearance of p-nitrobenzoic acid is yellow-white crystals. p-Nitrobenzoic acid is soluble in ethanol, ether, chloroform, acetone, boiling water, slightly soluble in benzene and carbon disulfide, and insoluble in petroleum ether[1]. P-Nitrobenzoic acid is an intermediate in organic synthesis of medicines, dyes, veterinary drugs, and photosensitive materials[2]. For the production of procaine hydrochloride, procainamide hydrochloride, p-aminomethyl benzoic acid, folic acid, benzocaine, cough, cephalosporin v, p-aminobenzoyl glutamic acid, benzyl Neil, as well as the production of reactive brilliant red M-8B, reactive red violet X-2R and filters, color film couplers, metal surface rust removers, sunscreens, etc. Melting point 239-241℃, relative density 1.61(20/4°C). Combustible. Soluble in ethanol, acetaldehyde, chloroform, acetone, boiling water, slightly soluble in benzene, carbon disulfide, insoluble in petroleum. Can be sublimated.

Picture 1 P-Nitrobenzoic acid powders
Experimental preparation
From the side chain oxidation of p-nitrotoluene, oxidation reaction is a strong exothermic reaction, and is a polyphase reaction, so must use stirring device and reagent (concentrated sulfuric acid) drop method, in order to avoid too high reaction temperature. In addition, a little water is added to the reactor first, so that the solid reactants can be wetted to increase the reaction contact surface, and the reaction can be moderated to prevent local reactions from being too violent.
In the post-treatment of crude products, because the crude products of p-nitrobenzoic acid contain unreacted p-nitrotoluene and name salt and other impurities, adding NaOH first can make the product p-nitrobenzoic acid into sodium salt and enter the solution, the impurities are separated by filtration method, and then the product is acidified.
The first step is to install the experimental equipment. Add 3g of ground p-nitrotoluene,9g sodium dichlorate and 11ml water to the 100ml three-way flask in the unit and turn on the mixer. Add 15ml concentrated sulfuric acid into the drop funnel, open the drop funnel, slowly add concentrated sulfuric acid, pay attention to strictly control the drop acceleration of concentrated sulfuric acid, so that the reaction mixture is higher than the boiling temperature (about 20-30min). After sulfuric acid is added, hollow plug or rubber plug is used to replace the drip funnel. After the reaction liquid is cooled slightly, it is heated on asbestos net with small fire, and the reactants are slightly boiled for 30min. Stop heating, slowly add 38ml cold water to the reaction solution after cooling, and close the agitator. Filtrate the mixture and place the crude solids in a small jar. The second step is to put the solid crude product into a 100ml beaker, add 38ml 5% sodium hydroxide solution, heat the crude product on asbestos net with low fire (not more than 60℃) to dissolve the crude product. After cooling, filtrate and discard the filter residue. Pour the filtrate slowly into another beaker containing 30ml of 15% sulphuric acid. Stir with a glass bar while pouring. Light yellow solids will be separated out. The filter cake was washed with a small amount of water until it was neutral and drained. The coarse product was recrystallized with 50% ethanol to obtain light yellow needle-like crystals.
Industrial Synthesis of p-Nitrobenzoic Acid
It is obtained by the oxidation of p-nitrotoluene. The oxidant can use sodium dichromate, air, manganese ore powder, nitric acid and so on. Using p-nitrotoluene as a raw material, in the presence of sulfuric acid, the oxidation reaction is carried out with sodium dichromate at 55°C to generate p-nitrobenzoic acid. The reaction solution is filtered, centrifugally dehydrated, washed with water and dried to obtain the finished product. In addition, using the dextrorotate 1-p-nitrophenyl-2-amino-1,3-propanediol in the production of chloramphenicol, it is easy to oxidize p-nitrobenzoic acid with nitric acid.
Storage of p-nitrobenzoic acid
p-Nitrobenzoic acid should be sealed, dry and protected from light. Production equipment should be sealed to prevent leakage. Operators should wear protective equipment. Packed in iron drums, fiberboard drums or sacks lined with plastic bags. The net weight of each barrel (bag) is 20kg or 40kg. Store in a cool, ventilated, dry place, protected from heat and sun. Storage and transportation according to the regulations of toxic chemicals.
Reference
1 Wang Qunwei, Wei Yunyang, Yue Caibo. Preparation of 3-methyl-4-nitrobenzoic acid by phase transfer catalytic oxidation. 2007
2 Yue Caibo, Wei Yunyang, Qiu Shuifa, etc. Catalytic oxidation of molecular oxygen to synthesize 3-methyl-4-nitrobenzoic acid. "Applied Chemistry", 2005
Related articles And Qustion
See also
Lastest Price from 4-Nitrobenzoic acid manufacturers

US $1.70-1.55/kg2025-08-07
- CAS:
- 62-23-7
- Min. Order:
- 1000kg
- Purity:
- 99%
- Supply Ability:
- 50 tons
US $0.00/kg2025-08-05
- CAS:
- 62-23-7
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 20 tons