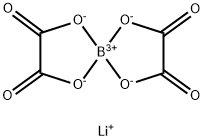
The role of Lithium bis(oxalate) borate as electrolyte additives
Description
Lithium bis(oxalate) borate, LiB-(C2O4)2 (LiBOB), is one of the most widely used electrolyte additives in Li-ion batteries and has the potential to be an alternative for the commercially used lithium hexafluorophosphate (LiPF6) as an electrolyte salt when dissolved in suitable solvents[1]. LiBOB has received great interest owing to its superior properties, such as good solubility in organic solvents, improved cycling stability with high voltage cathodes, environmentally friendliness, and low cost.
Use and mode of action
LiBOB provides electrochemical stability at high applied potentials and thermal stability under various electrochemical conditions. LiBOB also promotes forming a protective solid−electrolyte interface (SEI) layer, thus increasing capacity and cycle life when used as an electrolyte additive.
In addition, LiBOB is a promising organic lithium salt and a reactant for crosslinking due to the coordination and catalytic action of the BOB− group. A small amount of PC is used to facilitate the dissociation of LiBOB and improve the interfacial properties without impairing the aggregation behavior of the plasticized polymer electrolyte. SPPG is the polymer matrix in the as-synthesized polymer electrolyte, while LiBOB and PC are frequently used as lithium salt and solvent, respectively. In other words, the absence of additives produces a minimum amount of residue[2].
LiBOB can address the need for stable electrolyte compositions for high-voltage cathode materials and applications. In a mixed solvent system (γ-butyrolactone and dimethyl carbonate), it was shown that LiBOB provides oxidative stability up to 5.3 V in Li-ion batteries, whereas LiPF6 can be only employed up to 4.5 V. The thermal stability of bulk LiPF6 is 80 °C, and it is safe to use up to 50 °C in LIBs. LiPF6 is susceptible to decomposing at higher temperatures into LiF and PF5, resulting in low electrochemical performances and safety issues. On the other hand, bulk LiBOB decomposes at 300 °C and is stable up to 70 °C in LIBs. Several other reports show that LiBOB enables stable operation at voltages higher than 4.5 V. Besides this, LiBOB provides a safer operation for Li-ion batteries due to their high thermal stability and their role in protecting SEI formation. SEI layer formation plays an active role in the first cycle of the battery and keeps growing and changing with processes such as dissolution and precipitation.
References
[1] Ceren Zor. “Guide to Water Free Lithium Bis(oxalate) Borate (LiBOB).” The Journal of Physical Chemistry C 125 21 (2021): 11310–11317.
[2] Jianing Li. “Prospective Application, Mechanism, and Deficiency of Lithium Bis(oxalate)Borate as the Electrolyte Additive for Lithium-Batteries.” Advanced Energy Materials 13 35 (2023).
You may like
See also
Lastest Price from Lithium bis(oxalate)borate manufacturers

US $0.00/KG2025-09-25
- CAS:
- 244761-29-3
- Min. Order:
- 1KG
- Purity:
- 98% min
- Supply Ability:
- 200T

US $0.00/kg2025-08-21
- CAS:
- 244761-29-3
- Min. Order:
- 1kg
- Purity:
- 99%min
- Supply Ability:
- 20tons