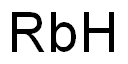The applications of Rubidium
Rubidium ,chemical symbol Rb, atomic number 37, and relative atomic mass (i.e., atomic weight) 85.4678(3), is the fourth alkali metal of group IA(1) of Mendeleev’s periodic chart. The word rubidium comes from the Latin rubidus, owing to the very deep red color of its chief atomic spectra line.

Properties
Rubidium is a soft, ductile, and malleable metal, silverywhite when freshly cut, and with a low density (1532 kg.m–3) between that of sodium and magnesium (it is the fourth lightest element). It has a body-centered cubic crystal lattice structure. Owing to its low melting point (m.p. 39.05°C), it can be liquid at room temperature. It decomposes water vigorously and also reacts with ice, even at –108°C, evolving hydrogen gas and forming the caustic rubidium hydroxide, RbOH.
It ignites spontaneously in oxygen and hence is difficult to handle because it reacts spontaneously in air. Like the other alkali metals, it combines vigorously with mercury, forming a stable rubidium amalgam.
Rubidium and cesium are miscible in all proportions and have complete solid solubility; a melting-point minimum of +9°C is reached. Because of the higher specific volume of rubidium compared with the lighter alkali metals, there is less tendency for it to form alloy systems with other metals. Owing to its more negative standard electrode potential (i.e., 2.924V/SHE), it is the second most electropositive element after lithium. It colors the flame of a Bunsen gas burner violet (424 nm).
Occurrence
Rubidium is widely distributed in nature. Its abundance in the earth’s crust is estimated to be 90 mg/kg. Rubidium occurs at trace levels in many potassium minerals. Often it is associated with cesium.
Some rubidium-containing minerals are lepidolite, leucite, petalite, feldspars, pollucite, beryl, and amazonite. The metal is never found as a major constituent in any mineral. Rubidium also occurs in many rocks such as basalts, granites and clay shales. Rubidium is found in seawater at an average concentration of 0.12 mg/L.
Applications
Rubidium is little used outside research. It has been used as a component of photocells, to remove traces of oxygen from vacuum tubes and to make special types of glass.
It is easily ionised so was considered for use in ion engines, but was found to be less effective than caesium. It has also been proposed for use as a working fluid for vapour turbines and in thermoelectric generators.
Rubidium nitrate is sometimes used in fireworks to give them a purple colour.