The preparation of polyethyleneimine
Introduction
Polyethyleneimine (PEI), an organic polyamine polymer, is one of the most prominent examples of cationic polymers capable of gene transfection in vitro and in vivo into various cell lines and tissues[1]. PEI was also applied in different fields from gene therapy and several studies have emphasized the importance of this polymer in medicinal chemistry. Since the first successful PEI-mediated oligonucleotide transfer conducted by the group of Jean-Paul Behr, PEI has been derivatized to improve the physicochemical and biological properties of polyplexes. Several PEI transfection agents have been made commercially available, including ExGen500 and jetPEI.

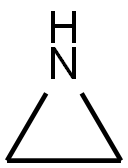
Picture 1 Polyethyleneimine polymer
The preparation of polyethyleneimine
Polyethyleneimine (PEI) is among the earliest and most widely studied cationic polymers for gene delivery, including the delivery of RNA. It has high gene transfection efficiency and is often referred to as the gold standard for non-viral gene transfection. PEI can be in either linear or branched structures and its positive charge is conferred by numerous amine groups separated by short alkyl spacers, which lead to very high positive charge density within its structure. The linear PEI can be synthesized via ring-opening polymerization of 2-ethyl-2-oxazoline followed by acid-catalyzed hydrolysis, resulting in a linear structure with only secondary amines in the polymer backbone and primary amines in the terminal. The branched PEI is synthesized by ring-opening polymerization of aziridine, which gives rise to the branched architecture with primary, secondary and tertiary amines at a nearly 1:2:1 ratio. It has been reported that the degree of polymerization, branching as well as charge density of PEI strongly affected its transfection efficiency, but a concrete structure-function relationship has yet to be concluded. The high RNA transfection efficiency of PEI could be attributed to strong positive surface charge that is able to condense RNAs into cationic nano-complexes, which promotes interaction with the negatively charged cell membrane, facilitates cellular endocytosis and also protects RNAs from enzymatic degradation; large number of amine groups that can be protonated at decreased pH in endosomes, which induces the “proton sponge” effect and causes an influx of counter ions into the endosomes, eventually leading to endosomal rupture and release of RNAs to the cytoplasm. Although PEI shows high RNA transfection efficiency, it is accompanied by pronounced toxicity and adverse effects arising from cation-induced membrane destabilization, nonspecific protein interaction, and immune response, limiting its application and translation to the clinics. Low-molecular-weight PEIs were reported to transfect siRNA and mRNA with improved toxicity profiles, however, their transfection efficiency was often compromised and biocompatibility concerns persisted.
Structure
PEI exists as both a branched and linear structure. Branched PEI (bPEI) is synthesized via acid-catalyzed polymerization of aziridine, whereas the linear structure (lPEI) is synthesized via ring opening polymerization of 2-ethyl-2-oxazoline followed by hydrolysis. PEI-derived vectors have been used to deliver oligonucleotides, plasmid DNA (pDNA), and Epstein-Barr virus (EBV) DNA, as well as RNA and intact ribozymes.
The mechanism of PEI-mediated gene delivery
The mechanism of PEI-mediated gene delivery has been illustrated in many literature studies. As a polycation, PEI will spontaneously adhere to and condense DNA to form spherical complexes that are readily endocytosed by cells[2]. These complexes interact with the cell membrane and are endocytosed. PEI that has amine groups with low pKa values has been shown to exhibit ‘proton sponge’ character. When the complex is endocytosed by endosome, PEI is capable of buffering the endosomal vesicle, which leads to endosomal swelling and lysis, so that DNA can be released into the cytoplasm. Vesicle transport may depend on the cytoskeleton, and PEI/DNA complexes also travel along cytoskeletal tracks in order to contact the nucleus. The polyplex is subsequently translocated into the nucleus, followed by decondensation and separation of the DNA from the polycationic delivery vehicle, either outside or inside the nuclear membrane. The released DNA subsequently undergoes transcription and translation, giving rise to the protein product.
The efficacy of bPEI-derived vectors and their cytotoxicities strongly depend on material characteristics and polyplex properties, such as molecular weight, degree of branching, cationic charge density, buffer capacity, DNA content, particle size, and zeta potential. Moreover, it is considered that transfection efficacy is also influenced by experimental conditions, like the polyplex concentration, the presence or absence of serum during transfection, the incubation time, and the transfection model chosen for the gene delivery experiment.
PEI derivatives
Godbey et al. reported that the cytotoxicity of PEI derives from two mechanisms. Free PEI can cause cell death prior to cellular internalization by membrane destabilization. Alternatively, 7–9 h after cellular internalization (when DNA has been released from the complex), free PEI can induce cellular stress responses such as endothelial cell activation. In an effort to minimize the latter process, analogues that break down into less toxic, low molecular weight structures after cellular uptake have become appealing. Synthesis of biodegradable PEI compounds involved either the incorporation of reducible disulfide linkages or ester conjugation. Lee et al. synthesized reducible PEI derivatives by treatment of low molecular weight PEI (800 Da) with either dithiobis (succinimidylpropionate) or dimethyl-3, 3′-dithiobispropionimidate. Sun et al. synthesized the reducible SS-PEI by Michael addition between cystamine bisacrylamide and low-molecular-weight branched 800 Da PEI. Peng et al. (2009) have prepared disulfide crosslinked polyethyleneimines (PEIX-SSY, where X refers to the molecular weight of raw PEI, and Y refers to the thiolation degree) in two steps: first, thiol groups were introduced on a raw polyethyleneimine (PEI) by the amine-induced ring-opening reaction of thiirane. Second, thiol groups were oxidized by dimethyl sulfoxide (DMSO) to form the disulfide cross-links. In addition to disulfide linkages, PEI derivatives with acid-labile ester linkages have been explored by several research groups to create biodegradable gene carriers.
Reference
1 X.-Z. Zhang, ... R.-X. Zhuo, in Bioactive Materials in Medicine, 2011
2 Xingya Jiang, ... Jinjun Shi, in Reference Module in Materials Science and Materials Engineering, 2021
Related articles And Qustion
See also
Lastest Price from POLYETHYLENEIMINE manufacturers

US $0.00/G2025-04-21
- CAS:
- 9002-98-6
- Min. Order:
- 5G
- Purity:
- 98%min
- Supply Ability:
- 30KG/month

US $10.00/ASSAYS2025-04-21
- CAS:
- 9002-98-6
- Min. Order:
- 1ASSAYS
- Purity:
- 99%
- Supply Ability:
- 100 mt