The preparation of cyanuric acid
Introduction
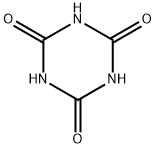
Cyanuric acid, also known as cyanuric acid, chemical name is 2,4,6-trihydroxy-1,3,5-triazine; 2,4,6 trihydroxy-s-triazine (2,4,6-triazinettriol) ; sym-triazinetriol. White crystals, separated from water, with two crystal waters. The taste is slightly bitter, basically non-toxic, soluble in hot water, slightly soluble in cold water, the aqueous solution is acidic, soluble in the aqueous solution of potassium hydroxide and sodium hydroxide, insoluble in cold organic solvents such as alcohol, ether, and benzene. The chemical formula is C3H3N3O3, and there are two isomers, namely ketone and enol structures, which usually exist as a mixture of two isomers. Cyanuric acid is mainly used in the manufacture of chlorinated derivatives for the synthesis of cyanuric acid formaldehyde resins, epoxy resins, herbicides, etc.

Picture 1 Cyanuric acid powders
property description
White crystals. About 330 ℃ depolymerization into cyanic acid and isocyanic acid. Those precipitated from water contain 2 molecules of crystal water, with a relative density of 1.768 (0°C), which loses moisture in the air and is weathered; those precipitated from concentrated hydrochloric acid or sulfuric acid are anhydrous crystals. 1g can be dissolved in about 200ml of water, odorless, slightly bitter taste. The product also exists in the form of ketone (or isocyanuric acid).
Dissolution
Soluble in hot water; hot alcohol; pyridine; concentrated hydrochloric acid and sulfuric acid without decomposition, also soluble in sodium hydroxide and potassium hydroxide aqueous solution, insoluble in cold alcohol; ether; acetone; benzene and chloroform.
preparation or source
Cyanuric acid can be obtained by the polymerization of urea. Mix urea with ammonium chloride, heat and melt, stir and heat up to 210°C and the solution thickens, heat up to above 230°C and the melt gradually solidifies, stir evenly, continue to heat up to 250°C, and keep the temperature for 15min. Cool to 100°C, soak in a small amount of water, drop to room temperature, soak in water, pulverize, and filter out solids. Add water and hydrochloric acid to the solid, stir and heat to 110°C, keep for 3h, add hydrochloric acid and water in stages, cool to 30°C, wash with water until neutral, filter, wash the filter cake with water, and dry to obtain the finished product. The purity of industrial products is ≥95%, and the consumption of urea per ton of products is 1200kg. Melamine is hydrolyzed with hydrochloric acid to obtain cyanuric acid, which is filtered, separated and washed to obtain fine products.
It can also be prepared by a half-cycle method. Put the cyanuric acid powder into the rolling ball, gradually add urea, its molar ratio is 1.7~2, heat up to 190 ℃, continue for half an hour, stop heating, and continue rolling until no ammonia gas is generated. A small part is taken out, pulverized, dissolved in sulfuric acid, boiled, centrifuged, filtered, washed and dried to obtain the finished product. The rest of the cycle continues to roll the ball.
use
Used in the synthesis of chlorinated derivatives, trichloroisocyanuric acid; sodium or potassium dichloroisocyanurate; used in the synthesis of cyanuric acid-formaldehyde resins; epoxy resins; antioxidants; coatings; adhesives; pesticides Herbicides; metal cyanide corrosion inhibitors; polymer material modifiers, etc.; used in the production of pharmaceutical halotrihydroxyzine. Manufacture of cyanuric acid chloride, paints, coatings, salts, lipids; mainly used in the synthesis of new bleaching agents, antioxidants, paint coatings, agricultural herbicides, and metal cyanide corrosion inhibitors, can be used for swimming pool chlorine stabilizers , sterilization, decontamination; can also be directly used in nylon, secco, flame retardant and cosmetic additives, etc.
other
It loses crystal water when heated to 150℃. Depolymerization occurs when heated to form cyanic acid. It reacts with phosphorus pentachloride to generate cyanuric chloride.
Cyanuric acid is digested by ruminants and is therefore widely added to animal feed. FDA stipulates the highest content, cyanuric acid + triuret <30%
Reference
1 Seifer G B. Cyanuric acid and cyanurates[J]. Russian Journal of Coordination Chemistry, 2002, 28(5): 301-324.
Related articles And Qustion
See also
Lastest Price from Cyanuric acid manufacturers

US $10.00/KG2025-06-26
- CAS:
- 108-80-5
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt

US $120.00/kg2025-04-21
- CAS:
- 108-80-5
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 1000000