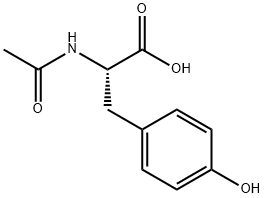
The preparation method of N-acetyl-L-tyrosine
N-acetyl-L-tyrosine is a derivative of L-tyrosine, an amino acid that builds neurotransmitters crucial for brain health. It may boost cognition and alertness in stressful situations.

Preparation method of N-acetyl-L-tyrosine
First, use Acetyl Chloride 98Min. acidulate in NaOH solution, temperature maintenance is kept the pH value at 9-10 at 5 degrees, after acidulate is finished, is acidified to the Congo red stain orchid with concentrated hydrochloric acid. Vacuum-evaporation gets gluey residue (Acetyl tyrosine crude product) to do. With ebullient acetone extracting crude product, repeat several, merging acetone extract filters, and is evaporated near dry, gets crystalloid Acetyl tyrosine behind the adding Sherwood oil. Be dissolved in hot ethyl acetate, evaporated to a small volume, add Sherwood oil, and get crystallization. Use petroleum ether, the dry Acetyl tyrosine that gets.
Then, the deacetylated product was obtained, acidified by adding concentrated hydrochloric acid, and precipitated, adding sodium hydroxide solution dissolving, the hydrolysis of 2N with the acetylize in the sodium hydroxide solution of the 2.5N of 17.6 times (V/W) of 1: 4-mole diacetyl oxide. Add the neutralization of 2N sulfuric acid, and be evaporated to dry. The last residue repeats to merge the acetone extract several times with the extracting of ebullient acetone, filters, and acetone evaporated is fallen, and the last syrupy shape material absorbs with minimum water, and crystallization by cooling gets N-acetyl-L-tyrosine.This method of acylation process can produce the acetic acid sodium salt, and crude product purity is low, and the solvent load is big, has the problem of solvent recuperation, drops into bigger; Solvent does not reclaim and then can cause the pollution to the environment.
Optimization of the preparation method of N-acetyl-L-tyrosine
The preparation method of N-acetyl-L-tyrosine involves using L-tyrosine in a water-based medium with the acylating agent diacetyl oxide. The process requires acetylation under temperature conditions of 50-80 ℃ for 45-75 minutes. The resulting product is Acetyl tyrosine crude, which is concentrated and treated with acid to obtain the crude product. After dissolving the crude product in hot water (75-80 ℃), decolorization, filtration, and post-crystallization, the final product is dried.
The present method uses water instead of a dilute alkaline solution in an acylation reaction, and the acylation process does not involve the formation of acetic acid sodium salt. The resulting crude product of N-Acetyl-L-tyrosine has high purity, which facilitates subsequent refinement with an acidulate rate of 95%. The refining process utilizes acetone as a solvent and hot water to dissolve and refine the crude product, which is environmentally friendly and harmless. The absence of inflammable and explosive substances improves production safety, and the total yield of products reaches 78-80%.
The raw materials and auxiliary materials used in this method are easy to obtain and inexpensive, and the process is simple and safe with a short technical process. Moreover, there is no production of waste salt, and the three waste products are easy to process.
You may like
Related articles And Qustion
See also
Lastest Price from N-Acetyl-L-tyrosine manufacturers

US $1.00/KG2025-08-02
- CAS:
- 537-55-3
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10ton/month

US $10.00/KG2025-04-21
- CAS:
- 537-55-3
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt