The Multifaceted Role of L-Tryptophan and Its Derived Natural Products
General Description
L-Tryptophan (L-Trp) is a large neutral amino acid (LNAA) present in living organisms, precisely one of the 20 L-amino acids (AAs) incorporated in proteins during the process of mRNA translation. All Trp residues in protein and peptide sequences are conventionally indicated with the alphabetic letter W. The AA L-Trp, discovered by the English chemist F. Hopkins in 1901, is also one of the 9 essential AAs for humans which cannot be endogenously synthesized and need to be supplied with aliments, as revealed through diet manipulation studies.
Besides being an intermediate of protein/peptide synthesis and turn-over, L-Tryptophan is the object of scientific investigations in human biological research since decades because of its transformation, after absorption, into a series of small bioactive, pleiotropic compounds, each capable of influencing a number of cell metabolic pathways and physiological responses. Hence, alterations of L-Trp-deriving compounds can be found associated with a variety of metabolic diseases and syndromes affecting those systems and organs responsible for maintaining the chemical, cellular, and behavioural homeostasis: the gut-liver apparatus and the neuroendocrine and immune systems along with the CNS. In particular, an imbalanced metabolism of this AA can interfere with the ability of these systems to interact with as well as discriminate, during development, stressors and stimuli, exogenous and endogenous antigens, and nutrients and xenobiotics.1

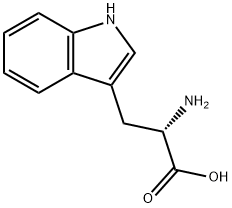
Figure 1. L-Tryptophan
Overview
Unique Structural Characteristics
The molecular evolution of life in Earth has selected the chemical structure of –R groups of the 20 L-AAs as the most suitable for building proteins. L-Tryptophan is the only AA in proteins deriving from indole, a bicyclic ring formed by a benzene and a pyrrole group, linked to the α-carbon by a –CH2-group. The presence of the indole ring in the chemical structure of Trp gives high hydrophobic features to this molecule among all protein AAs. Several AAs could be theoretically synthesized starting from indole, but, amongst these, only L-Tryptophan has been “retained” as a constituent of proteins in living organisms, presumably being the most simple structure of all possible indole AAs. In fact, Trp is the AA at the highest number of C atoms (C11) and the presence of other C atoms or substituent groups would be unnecessary. The advantage to keep indole in life chemistry derives either from the possibility to exploit its C11 skeleton in metabolism or to utilize it as –R residue in proteins and peptides to promote and stabilize their structure.1
Role in Biochemistry
Also, L-Tryptophan is metabolized to produce biologically active indole compounds which have great impact on life functions. In fact, beside being present in the chemical structure of the neurotransmitter 5-HT and, in turn, in the circadian molecules NAS and MLT in animals and humans, the indole ring of Trp can be transformed into bioactive compounds also by plants: for instance, the plant hormone indole-3-acetic acid (IAA) or auxin, the defense compounds indolyl glucosinolates, and the indole alkaloid and natural hallucinogen dimethyltryptamine. In particular, the plant hormone auxin has been found linked to a specific Trp metabolism pathway involved in plant photoperception and development.1
L-Tryptophan-derived Natural Products
Monomeric psilocybin and its congeners
The isolation and structure elucidation of psilocybin was first described in the late 1950‘s by Albert Hofmann and colleagues at Sandoz Laboratories.4 They used [β-14C]-l-tryptophan for investigative experiments that proved psilocybin's origin from this building block. Less than a decade later, Agurell and Nilsson utilized the same concept, used various radiolabeled precursors and presented a sequence of biosynthetic events which metabolize l-tryptophan to psilocybin. These authors proposed a five-step biosynthesis via decarboxylation to tryptamine as the initial step, followed by two methyltransfers, then 4-hydroxylation, and 4-O-phosphorylation as the terminal step. Recent work identified the genes encoding psilocybin biosynthesis enzymes in Psilocybe and other genera. The metabolic pathway was shown by the activity of four Psilocybe cubensis enzymes (PsiD, PsiH, PsiK, and PsiM), which provide l-tryptophan decarboxylase, indole-4-monooxygenase, kinase, and N-methyltransferase activity, respectively.2
Psilocyl Oligomers
Psilocybin-containing mushrooms instantly develop a blue hue when the mycelium is injured, or as they age. For decades, this iconic phenomenon has intrigued chemists and amateur mycologists alike. Oligomerization converts the l-tryptophan-derived indole nucleus into a blue chromophore. 2
β-Carbolines
β-carbolines, primarily harmane and harmine, have long been established as indirect neuroactive compounds as they strongly and reversibly inhibit monoamine oxidase isoenzyme (MAO)-A. This flavin-dependent enzyme is involved in the breakdown of serotonin and other neuroactive amines by oxidative deamination. Consequently, MAO A inhibition enhances their effect and prevents the bioactivity from being lost. MAO-A‘s substrate spectrum also includes psilocin, which aside from renal elimination, is eliminated by formation of 4-hydroxyindol-3-yl-acetaldehyde. β-carboline-mediated MAO-A inhibition consequently would intensify the effects of psilocin. We therefore encounter an intriguing, and in the fungal arena, unique scenario: one precursor, L-tryptophan, feeds into two different biosynthetic routes which result in metabolites that act on dissimilar targets (receptor versus catabolic enzyme), yet act synergistically by contributing to the same pharmacology. Mechanistically, this scenario is somewhat reminiscent of the natural products of the soil bacterium Streptomyces clavuligerus. It produces cephamycin, a β-lactam antibiotic, and simultaneously, clavulanic acid, i. e., an inhibitor of β-lactamase that could otherwise inactivate cephamycin. However, these biosyntheses do not involve L-tryptophan. 2
References:
[1] L. PALEGO. Tryptophan Biochemistry: Structural, Nutritional, Metabolic, and Medical Aspects in Humans[J]. Journal of Amino Acids, 2016, 96 1: 269-272. DOI:10.1155/2016/8952520.[2] CLAUDIUS LENZ. Taking Different Roads: l-Tryptophan as the Origin of Psilocybe Natural Products[J]. ChemPlusChem, 2020, 86 1: 1-205. DOI:10.1002/cplu.202000581.
You may like
Related articles And Qustion
Lastest Price from L-Tryptophan manufacturers

US $5.00/kg2025-11-03
- CAS:
- 73-22-3
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 300tons

US $0.00-0.00/Kg/Drum2025-04-21
- CAS:
- 73-22-3
- Min. Order:
- 1KG
- Purity:
- 98.5%-101.5%
- Supply Ability:
- 10 TONS