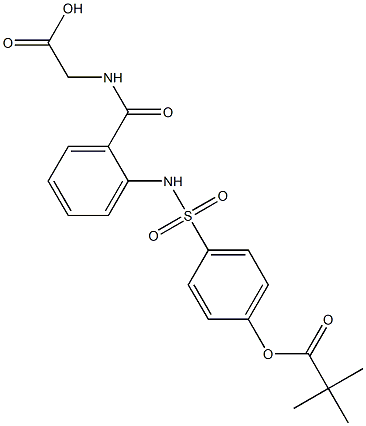
The introduction of Unii-fys6T7F842
General description
The Unii-fys6T7F842, with the CAS No: 331731-18-1, is also known as Immunoglobulin G1, anti-(human tumor necrosis factor) (human monoclonal D2E7 heavy chain), disulfide with human monoclonal D2E7 light chain, dimer. This chemical’s molecular formula is C6428H9912N1694O1987S46. Unii-fys6T7F842 injection is the first human anti-tumor necrosis factor approved for marketing in the world ɑ (TNF-ɑ) Monoclonal antibodies, first developed by American pharmaceutical companies, were first approved by the US FDA in 2002. It can specifically interact with soluble human TNF-ɑ Binding and blocking its interaction with TNF receptors p55 and p75 on the cell surface, thus effectively blocking TNF-ɑ Inflammatory effect of. In addition, Unii-fys6T7F842 may also bind transmembrane TNF-ɑ,It can induce apoptosis and other effects, and eliminate some pathogenic target cells. With the continuous expansion of indications, this variety quickly created a miracle in drug sales. Since 2012, Unii-fys6T7F842 has been ranked the top of global prescription drug sales, replacing Lipitor as the new "drug king".
Figure 1 Commercial drug of Unii-fys6T7F842.
Bioactivities of Unii-fys6T7F842
Unii-fys6T7F842 has many bioactivities according to the previous reports [1-4]. It had significant efficacy in well-designed, placebo-controlled trials in patients suffering from rheumatoid arthritis, both as monotherapy and in combination with various disease-modifying antirheumatic drugs, including methotrexate. Unii-fys6T7F842 was generally well tolerated during both concomitant therapy with methotrexate or standard antirheumatic therapy and monotherapy. In addition, the radiographic progression of structural joint damage was significantly inhibited by Unii-fys6T7F842 and improved quality of life [1]. Furthermore, Unii-fys6T7F842 was significantly more effective than placebo and methotrexate at relieving the signs and symptoms of psoriasis after 16 weeks of treatment, as assessed by the percentage of patients achieving a 75% improvement from baseline in Psoriasis Area and Severity Index. Based on open-label extension studies of up to 2 years’ duration, the efficacy of Unii-fys6T7F842 was sustained over the long term [2]. In addition, Unii-fys6T7F842 can be applied in Crohn’s disease [3] and refractory pulmonary sarcoidosis [4].
Side effects of Unii-fys6T7F842
Its side effect profile is favorable when compared with traditional systemic treatments for these diseases. It does not require laboratory monitoring. The most common side effects of adalimumab are injection site reactions. Adalimumab increases the risk of rare serious infections. There is a two-fold risk of serious infections with the use of adalimumab, as reported in the Premier trial. This risk should not be minimized in this way. It should not be used during periods of active infection. Its most notable infectious complication is the reactivation of tuberculosis. Tuberculosis screening should be according to country standards and may or may not include purified protein derivative test or chest X-ray. Deep fungal and other serious and atypical infection can also be promoted by adalimumab. It has been associated infrequently with skin rashes. Rare side effects include: worsening or initiation of congestive heart failure, a lupus-like syndrome, a promotion of lymphoma, medically significant cytopenia, and worsening or initiation of a multiple sclerosis/neurological disease. There has been reported pancytopenia and elevated tandamines with the use of adalimumab, which suggest that laboratory monitoring blood counts and liver functions, at least intermittently, are useful. In patients with any of the foregoing problems, its use should be extremely carefully considered. Adalimumab is a useful medication which can be safely used if its side effects are recognized [5].
Detection of Unii-fys6T7F842
Serum Unii-fys6T7F842 (Adalimumab, ADL) concentrations can be measured by enzyme-linked immunosorbent assay (ELISA) using the commercial kit [6]. For example, with the commercial kit of Promonitor ADL, Proteomika SLU, Derio, Spain, briefly, microwell strips were precoated with anti-ADL-human Fab. The ADL present in patients serum bound to anti-adalimumab-antibody was detected using a horseradish peroxidase (HRP)-labeled secondary antibody. Results were plotted on a titration curve and expressed in μg/mL.
References
[1]Paraskevi et al. Adalimumab for rheumatoid arthritis. Expert Opinion on Biological Therapy. 2006, 6(12): 1349-1360.
[2]Croom & McCormack. Adalimumab In Plaque Psoriasis. American Journal of Clinical Dermatology. 2009, 10: 43–50.
[3]Van Assche et al. dalimumab in Crohn's disease. Biologics-rargets & therapy. 2007, 1(4): 355-365
[4]Minnis et al. Adalimumab for refractory pulmonary sarcoidosis. Irish journal of medical science. 2016, 185(4): 969-971
[5]Scheinfeld. Adalimumab: a review of side effects. Expert opinion on drug safty. 2005, 4 (4): 637-41.
[6]Marinari et al. Detection of Adalimumab and Anti-Adalimumab Levels by ELISA: Clinical Considerations. Drug development research. 2014, 75: S11-S14.
Related articles And Qustion
Lastest Price from Adalimumab manufacturers

US $0.00/G2025-04-21
- CAS:
- 331731-18-1
- Min. Order:
- 1G
- Purity:
- 98%min
- Supply Ability:
- 30kg/month
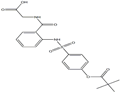
US $0.00-0.00/g2022-04-21
- CAS:
- 331731-18-1
- Min. Order:
- 10mg
- Purity:
- 99%
- Supply Ability:
- Adalimumab