The introduction of Tetrachlorantraniliprole
Description
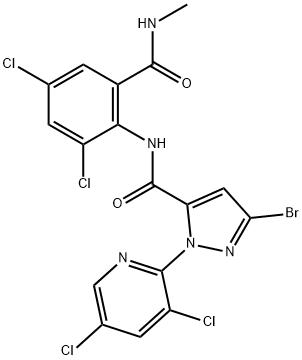
Tetrachlorantraniliprole (TCAP) is a novel anthranilic diamide insecticide targeting the ryanodine receptors of lepidopteran insect species with excellent insecticidal activity[1]. Tetrachlorantraniliprole was announced by Sinochem Corporation as a new insecticide in 2018 and has been developed under the code SYP9080.
Use
Tetrachlorantraniliprole belongs, just as the closely related market products chlorantraniliprole and cyantraniliprole, to the anthra-nilamide subclass of diamide insecticides, which are modulators of ryanodine receptors, hereby activating the calcium channels that cause an excessive release of intracellular calcium ions leading to muscle contraction failure. Similar to chlorantraniliprole, also tetrachlorantraniliprole seems to act specifically on lepidopteran species, such as Plutella xylostella (diamondback moth) and Pieris rapae (cabbage white butterfly) and is safe on non-target species, such as Harmonia axyridis (harlequin ladybeetle) and Eisenia fetida (redworm). TCTP has been registered in China for use on crops to control certain Lepidoptera, such as Cnaphalocrocis medinalis, Pyrausta nubilalis, and Spodoptera exigua[2].
![1H-Pyrazole-5-carboxamide, 3-bromo-N-[2,4-dichloro-6-[(methylamino)carbonyl]phenyl]-1-(3,5-dichloro-2-pyridinyl)- Article illustration](/NewsImg/2024-02-21/6384412015613502594144761.png)
Synthesis method
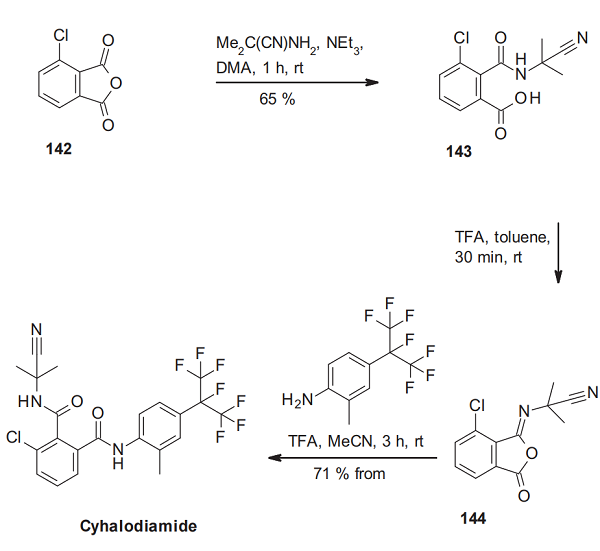
The synthesis of tetrachlorantraniliprole starts with replacing one of the two chlorine substituents next to the ring nitrogen of 2,4,6-trichlor pyridine (135) with hydrazine. The resulting dichlorinated 2-hydrazinopyridine 136 is then cyclized with diethyl maleate to the pyrazolinone derivative 137. Its conversion with phos- phorus oxybromide leads to the bromopyrazole derivative 138. Next, the ester function is transformed by saponification to the carboxylic acid 139 and subsequent chlorination to the acid chloride derivative 140[3]. Amide formation of 140 with 2-amino-3,5-dichloro-N-methylbenzamide delivers tetrachlorantraniliprole.
![1H-Pyrazole-5-carboxamide, 3-bromo-N-[2,4-dichloro-6-[(methylamino)carbonyl]phenyl]-1-(3,5-dichloro-2-pyridinyl)- Article illustration](/NewsImg/2024-02-21/6384412043586426259952143.png)
References
[1] Haiyuan Teng. “Evaluation of the sublethal effect of tetrachlorantraniliprole on Spodoptera exigua and its potential toxicity to two non-target organisms.” PLoS ONE (2020): e0242052.
[2] Zhou Lu , Hongyu Pan, Zhiguang Hou . “Degradation of anthranilic diamide insecticide tetrachlorantraniliprole in water: Kinetics, degradation pathways, product identification and toxicity assessment.” Science of the Total Environment 836 (2022): Article 155448.
[3] Stephane Jeanmart . “Synthetic approaches to the 2015–2018 new agrochemicals.” Bioorganic & Medicinal Chemistry 39 (2021): Article 116162.