How to synthesis Cyhalodiamide?
Description
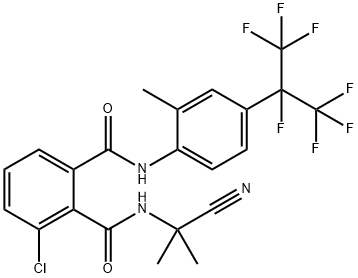
Cyhalodiamide (3-chloro-N2-1-methyl-1-cyano-ethyl-N1-[2-methyl-4-(1, 2, 2, 2-tetrafluoro-1-trifluoromethyl-ethyl)-phenyl]-phthalamide, ZJ4042) is a novel phthalic diamide insecticide which was independently developed by Zhejiang Research Institute of Chemical Industry, Ltd.
Uses
The action mode of cyhalodiamide relies on activating the ryanodine receptors (RyRs) and affecting the release of calcium, leading to feeding cessation, emesis, hunger, dehydration, and terminal death[1]. Cyhalodiamide exhibits high efficacy in targeting insects and low toxicity to mammals, and the persistent period and safety of cyhalodiamide showed excellent. Cyhalodiamide is mainly used to control Lepidoptera pests, including Cnaphalocrocis medinalis (Guenée), Chilo suppressalis (Walker), Scirpophaga incertulas (Walker), Pieris rapae (L.), Plutella xylostella (L.), Spodoptera exigua (Hübner), Prodenia litura (Fabricius), Helicoverpa armigera (Hübner), etc. Recently, it has been discovered that cyhalodiamide is also active against the nematode Bursaphelenchus xylophilus, the causal agent of the pine wilt.
Synthesis method
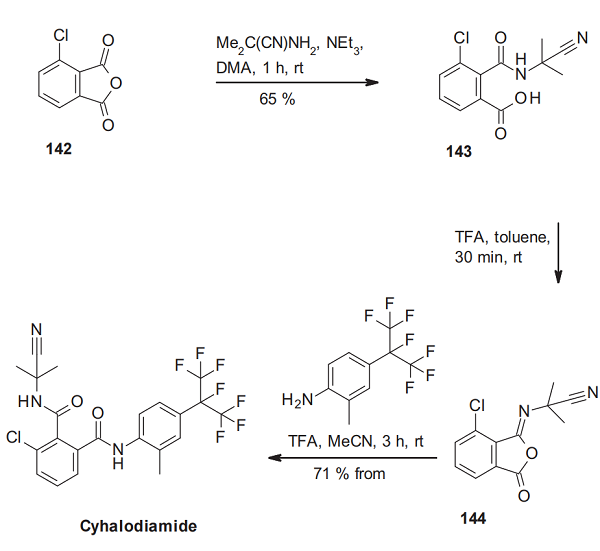
The synthesis of cyhalodiamide can be achieved by a three-step ring-opening ring-closure ring-opening sequence and starts the ring-opening of 3-chlorophthalic anhydride (142) with 2-amino-2-methylpropionitrile to the phthalic acid monoamide 143. Under acidic conditions, this phthalamic acid derivative cyclizes to the phthalic isoimide 144, which delivers cyhalodiamide via ring-opening with 2- methyl-4-heptafluoroisopropylaniline[2].
References
[1] Yiping Liu . “Distribution and degradation kinetics of cyhalodiamide in Chinese rice field environment.” Chinese Journal of Chemical Engineering 26 10 (2018): Pages 2185-2191.
[2] Stephane Jeanmart . “Synthetic approaches to the 2015–2018 new agrochemicals.” Bioorganic & Medicinal Chemistry 39 (2021): Article 116162.