The introduction of 7-Fluoro-imidazo[1,2-a]pyridine
General description
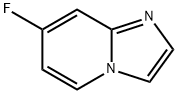
The 7-Fluoro-imidazo[1,2-a]pyridine, with the CAS No: 1260903-17-0, is also known as Imidazo[1,2-a]pyridine, 7-fluoro-. This chemical’s molecular formula is C7H5FN2 and molecular weight is 136.13. Its density is 1.27±0.1 g/cm3 (Predicted). The 7-Fluoro-imidazo[1,2-a]pyridine is a kind of synthetic intermediate. Its structure is as follows:
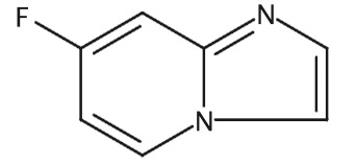
Figure 1 Structure of 7-Fluoro-imidazo[1,2-a]pyridine.
Chemical synthesis of 7-Fluoro-imidazo[1,2-a]pyridine
The 7-Fluoro-imidazo[1,2-a]pyridine can be synthesized by 3 steps according to the previous work [1]. Add 128g (1.0 mol) of 2-amino-4-chloropyridine, 1.28L of ethanol, 336g (4.0 mol) of sodium bicarbonate and 235g of 50% chloroacetaldehyde aqueous solution into a 3L three necked bottle with a magnetic stirring device at room temperature. After the addition, raise the temperature to 80 ° C for reflux and react for 2h; Cool the reaction to 20~30 ° C, concentrate, remove ethanol, add water and ethyl acetate in turn, separate the liquid, and concentrate the organic phase to obtain 136g brownish red oily liquid, with a yield of 90%. Add 100g (655 mmol) 7-chloroimidazolo [1,2-A] pyridine, 107g (688 mmol) p-methoxybenzyl chloride and 500mL N, N-dimethylformamide into a 1L three necked bottle with a magnetic stirrer, replace nitrogen, heat up to 90 ° C and stir for 2h; After the reaction, the temperature shall be reduced to 20~30 ° C, and the reaction liquid shall be directly put into the next reaction. Take a small amount of samples for separation, purification and characterization. The results of NMR structure identification are as follows:1HNMR (DMSO-d6400MHz) δ ppm 8.56(d,1H),7.90(d,1H),7·72(d,1H),7.42(m,2H),7.16(d,2H),6.82(d,2H),5.96(s,2H),3.80(s, 3H). Add 196g (1.3 mol) of cesium fluoride into the above reaction solution, replace nitrogen, raise the temperature to 100 ° C, stir for 1h, cool down to 20-30 ° C after the reaction, add water and n-butanol, stir for separation, and concentrate the organic phase to obtain 280 g of brown yellow solid, with a two-step yield of 91%. The NMR structure identification results are as follows: 1HNMR (DMSO-d6400MHz) δ ppm 8.55(d,1H),7.90(d,1H),7.72(d,1H),7.16(d,2H),7.09 (m,2H),6.82(d,2H),5.96(s,2H),3.80(s,3H). Add 100g (324mmol) of 7-fluimidazolo [1,2-A] pyridine quaternary ammonium salt and 300mL of methanesulfonic acid into a 1L three necked bottle with a magnetic stirrer, replace nitrogen, raise the temperature to 80 ℃, stir for 2h, cool down to 20-30 ℃ after the reaction, add water and ethyl acetate, add 50% sodium hydroxide to adjust the pH of the aqueous phase to 9-10, separate the solution, concentrate the organic phase, recrystallize with ethyl acetate n-heptane, and obtain 32.5g of light yellow solid, with a yield of 80%, The results of nuclear magnetic structure identification are as follows: 1HNMR (DMSO-d6400MHz) δ ppm 8.00 (t, 1H), 7.52 (d, 2H), 7.19 (d,1H), 6.61 (t, 1H).
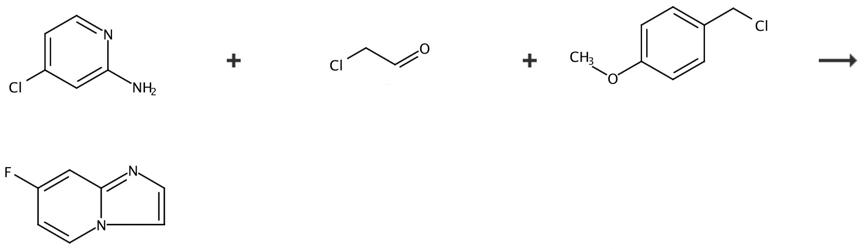
Figure 2 Chemical synthesis of 7-Fluoro-imidazo[1,2-a]pyridine.
Application of 7-Fluoro-imidazo[1,2-a]pyridine
7-fluoroimidazole [1,2-a] pyridine is an important intermediate in organic synthesis. It is widely used in the synthesis of pharmaceuticals, pesticides and fine chemicals [2]. 7-fluoroimidazole [1,2-a] pyridine can be used to synthesize dihydroindene amine compounds. These compounds or compositions containing these compounds have important applications in the treatment or prevention of diseases or disorders related to protein kinase activity or cell proliferation abnormalities [3]. In addition, 7-fluoroimidazole [1,2-a] pyridine can synthesize pyridine compounds suitable for antagonizing hematopoietic progenitor cell kinase 1 (HPK1), namely mitogen-activated protein kinase 1 (MAP4K1). Because of its role in promoting immunity and promoting immune evasion of tumor cells, these compounds are involved in the treatment of some autoimmune diseases and anti-tumor immunity [4].
References
[1]Wang et al. 7-Fluoro-imidazo[1,2-a]pyridine intermediate and its production method. CN112409354, 26 Feb 2021.
[2]Luo et al. Method for synthesizing 7-fluoroimidazo[1,2-a]pyridine promoted by copper complex. CN111635401, 08 Sep 2020.
[3]Xiao et al. Preparation of indan derivatives as protein kinase inhibitors. CN102584830, 18 Jul 2012.
[4]Kaila et al. Preparation of substituted pyrrolopyridinones as HPK1 antagonists and uses thereof. PCT Int. Appl., 2021050964, 18 Mar 2021.