The difference between sulfurous acid and sulfuric acid
Sulfuric acid
Sulfuric acid, or hydrogen sulfate, is a highly corrosive, clear, colorless, odorless, strong mineral acid with the formula H2SO4. It is also one of the top 10 chemicals the paper industry releases (by weight) [1]. In modern industry, sulfuric acid is a necessary commodity chemical used primarily for producing phosphoric acid. It is also suitable for removing oxidation from iron and steel, so it is used in large quantities by metal manufacturers. Sulfuric acid is very reactive and dissolves most metals; it is a concentrated acid that oxidizes, dehydrates, or sulfonates most organic compounds, often causing charring.
Sulfurous acid
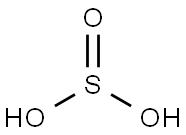
Sulfurous acid has the formula H2SO3 and a molecular weight of 82.075 g/mol. Its CAS number is 7782-99-2. No clear evidence exists that sulfurous acid exists in solution, but the molecule has been detected in the gas phase[2]. However, the conjugate bases of this elusive acid are common anions: bisulfite (or hydrogensulfite) HSO3− and sulfite SO3-. Bisulfite salts are typically prepared by treatment of alkaline solutions with excess sulfur dioxide: SO2 + NaOH ⇒ NaHSO3.
Differences
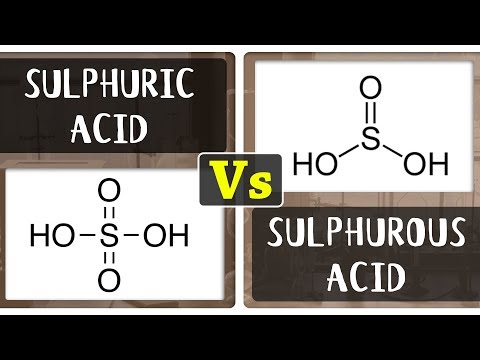
Sulfuric acid (H2SO4) and sulfurous acid (H2SO3) are inorganic acids containing Sulfur, hydrogen, and oxygen as elements. The critical difference between sulfuric acid and sulfurous acid is in the oxidation number of Sulfur. Moreover, Sulfuric acid is a strong acid, and sulfurous acid is weak. The chemical formula of sulfuric acid is H2SO4, where the oxidation number of Sulfur is +6. The geometrical structure of this molecule is tetrahedral. However, the chemical formula of sulfuric acid is H2SO3, where the oxidation number of Sulfur is +4. The geometrical structure of this molecule is trigonal pyramidal.
The oxidation state of S is more in H2SO4, which is +6 as compared to +4 in H2SO3. The greater the oxidation state of the central atom, the more it attracts the electrons towards itself, and also, the more it stabilizes the conjugate base.
At the point when two compounds have a similar overabundance of oxygen count, the next factor to consider is the electronegativity of the central atom. The more electronegative the central atom, the more it pulls out electron density from the acidic proton, expanding the acidity. So, we can say that electronegativity increases as we go left to right in the periodic table, and as the electronegativity increases, acid strength increases. That is why Sulphuric acid is a more vital acid than Sulfurous acid.
In addition, the rule says that the acid where Sulfur has the lower oxidation number must have the ending “ous” and that with a higher oxidation number must have the ending “ic” Therefore:
H2SO4 (ox. number = +6) —-> sulphuric acid
H2SO3 (0x. number = +4) —-> sulphurous acid
References
[1] Cheremisinoff, N. and P. Rosenfeld. “Sources of air emissions from pulp and paper mills.”Handbook of Pollution Prevention and Cleaner Production 2010: 179-259.
[2] Ropp, R. C. “Group 16 (O, S, Se, Te) Alkaline Earth Compounds.”Encyclopedia of the Alkaline Earth Compounds 2013:105-197.