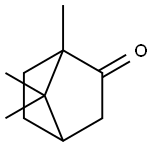
The chemical property and Lewis structure of Camphor
Overview
Camphor formula, also known as 2-Camphanone formula or Kampfer formula, is a bicyclic monoterpene ketone. Camphor is a waxy solid, usually white or transparent in the shape of crystals, with a molecular formula of C10H16O. Camphor is found in the woods of the camphor laurel tree, a large evergreen tree in Asia.
Uses
Camphor has a powerful aroma and is used in various ways, from cooking to being used as an herbal medicine. It is a terpenoid, a part of a big class of organic compounds with unsaturated molecules composed of linked isoprene units. It is also a cyclic molecule, meaning it is in a constant cycle.
chemical property
Camphor (C10H16O) is a cyclic molecule that occurs in cycles and is repeated regularly. Therefore, the Ax form of Camphor is cyclic. It has a molar mass of 152.23 g/mol. The boiling point is 339.2°F, and the melting point is 347°F. The boiling and melting points are relatively low compared to other molecules.
Lewis structure
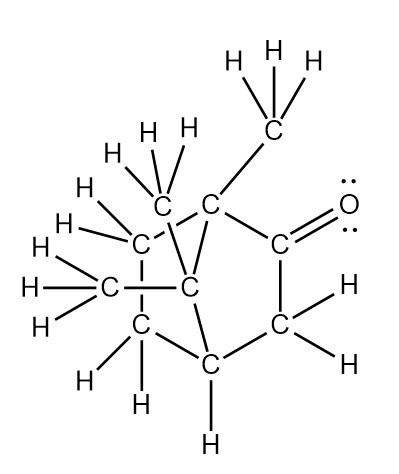
The Lewis structure of Camphor is shown above. As shown, each C and O atom has 8 electrons. In addition, only the O atom has two lone pairs of electrons around it.
You may like
Related articles And Qustion
See also
Lastest Price from Camphor manufacturers

US $0.00-0.00/KG2025-12-13
- CAS:
- 76-22-2
- Min. Order:
- 1KG
- Purity:
- 96%min,USP/BP
- Supply Ability:
- 10tons/month

US $3.00/box2025-09-28
- CAS:
- 76-22-2
- Min. Order:
- 1box
- Purity:
- 99%
- Supply Ability:
- 20tons per month