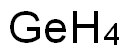The brief introduction of Germanium
Description
Germanium (Ge), a chemical element between silicon and tin in Group 14 (IVa) of the periodic table, is intermediate in properties between the metals and the nonmetals. It is a lustrous, hard-brittle, grayish-white metalloid in the carbon group, chemically similar to its neighbors, silicon and tin. Its atom electron configuration is [Ar]3d104s2 4p2. It occurs mainly in the oxidation state +4, although many +2 compounds also exist. Other oxidation states are rare: +3 is found in compounds such as Ge2Cl6, +3, and +1 are found on the surface of oxides, or negative oxidation states such as 4 in magnesium germanide, Mg2Ge.
Ge's abundance in the Earth’s crust is about 1.6 ppm. Only a few minerals like argyrodite (Ag8GeS6), briartite (Cu2(Zn, Fe)GeS4), germanite (Cu13Fe2Ge2S16) , and renierite ((Cu,Zn)11(Ge,As)2Fe4S16) contain substantial concentrations of Ge. Only a few of them (especially germanite) are, very infrequently, found in mineable amounts. About 118 tons of germanium were produced worldwide in 2011, mostly in China, Russia, and the United States. Germanium is produced mainly as a by-product from sphalerite (ZnS) ores where it can be present in concentrations as high as 0.3%, in particular from low-temperature sediment-hosted, massive Zn-Pb-Cu(-Ba) deposits and carbonate-hosted Zn-Pb deposits.
Minerals
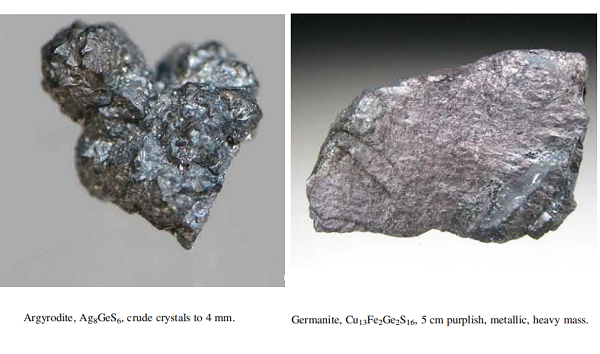
Thirty-two different minerals are known to contain germanium in their crystal structure. The sulfide class contains 16 different minerals, for example, argyrodite (Ag8GeS6), germanite (Cu13Fe2Ge2S16), and renierite (Cu11, Zn)11Fe4(Ge41, As51)2S16). Five different oxide class minerals are known, for example, argutite (GeO2), eyselite (Fe31Ge41 3 O7 OH ð Þ), and stottite (Fe21[Ge41(OH)6]). The sulfate class contains 4 different minerals with Ge, carraraite (Ca3(SO4)[Ge(OH)6](CO3) 12H2O), fleischerite (Pb3Ge(SO4)2(OH)6.3H2O), itoite (Pb3Ge41(SO4)2O2(OH)2), and schaurteite (Ca3Ge(SO4)2(OH)6.4H2O). A single phosphate is known to possibly contain Ge, galloplumbogummite (Pb(Ga, Al, Ge)3(PO4)2(OH)6). Six minerals are found in the silicate class, which also includes the germanates, for example, brunogeierite (Ge41Fe21 2 O4), carboirite (FeAl2(GeO4)O(OH)2), and krieselite ((Al, Ga)2(GeO4)(OH)2).
Isotopes
Ge exists in 5 natural isotopes: 70Ge, 72Ge, 73Ge, 74Ge, and 76Ge. Of these isotopes, 76Ge is very slightly radioactive, decaying by double β decay with a half-life of 1.78×1021 years. 74Ge is the most abundant isotope, with a natural abundance of 36.7%. 76Ge is the rarest isotope, with a natural abundance of 7.75%. Under bombardment with α particles, 72Ge will produce stable 77Se, releasing high-energy electrons. Due to this, it is applied together with radon for nuclear batteries.
In addition, at least 27 radioisotopes have been synthesized, ranging from 58Ge to 89Ge. The most stable of these radioisotopes is 68Ge, which decays via electron capture with a half-life of 270.95 days. The least stable radioisotope is 60Ge, with only a half-life of 30 milliseconds. However, a majority of the Ge radio-isotopes decay by β decay, 61Ge, and 64Ge decay by β1 delayed proton emission. 84Ge through 87Ge isotopes also show minor β2 delayed neutron emission decay paths.
You may like
Related articles And Qustion
See also
Lastest Price from Germanium manufacturers

US $2000.00-1600.00/kg2025-01-07
- CAS:
- 7440-56-4
- Min. Order:
- 10kg
- Purity:
- 99.99%
- Supply Ability:
- 1000kg per month

US $2000.00-1600.00/kg2025-01-07
- CAS:
- 7440-56-4
- Min. Order:
- 10kg
- Purity:
- 99.99%
- Supply Ability:
- 1000kg per month