The Application of Nickel Carbonate as Catalyst
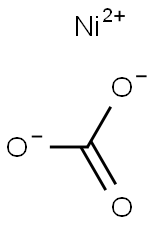
Nickel carbonate presents as a light green crystalline substance, exhibiting nearly insolubility (0.093 g/L) in water at 25°C, and remaining insoluble in hot water while readily soluble in acids. The predominant form of nickel carbonate is basic nickel carbonate (NiCO3· 2Ni(OH)2 · 4H2O), characterized by its water insolubility and solubility in ammonia and dilute acids. In nature, nickel carbonate tetrahydrate is found as zaratite.

Amorphous Nickel Carbonate Catalyst
Compared with crystalline catalysts, the feature of more unsaturated coordinated atoms and higher surface energy boosts the electrocatalytic performance of amorphous catalysts. Herein, an amorphous nickel carbonate, a urea oxidation reaction (UOR) catalyst, was synthesized via a facile precipitation approach[1]. The UOR catalysis on the as-synthesized amorphous nickel carbonate mainly follows the indirect chemical-oxidation path. When employed in UOR catalysis, the as-synthesized amorphous nickel carbonate decorated on glass carbon electrode delivers a current density of 117.69 mA·cm−2 at 1.6 V vs. RHE with a small Tafel slope of 40.7 mV·dec-1, which is comparable to the most active reported catalysts. This work proposes a facile method to synthesize an efficient amorphous nickel carbonate UOR catalyst.
Platinum and Nickel Carbonate Hydroxide Catalyst
Pt-based nanostructures are an indispensable component in the anodic electrocatalysts of direct methanol fuel cells (DMFCs). Unfortunately, they often suffer from severe operational durability issues, due to CO poisoning. Here, a ternary hybrid consisting of Pt nanocrystals, ultrathin nanoflower-like nickel carbonate hydroxide (NiCH) and porous carbon is designed and fabricated to overcome this issue[2].
Due to the abundance of defects and the large surface area of NiCH, water molecules readily dissociate to form OH adspecies. These species facilitate the oxidative removal of carbonaceous poisons from nearby Pt nanoparticles through the Langmuir-Hinshelwood mechanism. The incorporation of porous carbon addresses the poor conductivity of NiCH and enhances charge transfer. Notably, this hybrid electrocatalyst maintains over 39% of its initial activity after 5000 seconds of stability testing, with the peak current of the MOR reduced by only 25% after 8000 cyclic voltammetry cycles, demonstrating remarkable CO tolerance. CO stripping experiments further underscore this capability. Density functional theory (DFT) calculations provide additional insight, revealing that electronic interactions between H atoms from NiCH and Pt cause a downshift in the d-band center of Pt atoms, facilitating the release of CO adsorbed on its surface. This research presents a promising candidate for the development of anodic electrocatalysts in direct methanol fuel cells (DMFCs).
References
[1] An amorphous nickel carbonate catalyst for superior urea oxidation reaction. doi:10.1016/j.jelechem.2023.117856
[2] Insights into the synergy of platinum and nickel carbonate hydroxide for efficient methanol electro-oxidation. doi:10.1016/j.apsusc.2023.156587
You may like
See also
Lastest Price from Nickel carbonate manufacturers

US $6.00/kg2025-04-21
- CAS:
- 3333-67-3
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 2000KG/Month

US $0.00-0.00/KG2025-04-15
- CAS:
- 3333-67-3
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 500000kg