The application of Dexpanthenol
General Description
Dexpanthenol (International Nomenclature of Cosmetic Ingredients (INCI) name panthenol) is the alcohol corresponding to pantothenic acid (the water-soluble vitamin B5)[1]. It is the biologically active form of panthenol, which is seen as a D enantiomer. It is oxidized to pantothenic acid in the skin and mucous membranes. It stimulates the biosynthesis of glutathione, ATP, and coenzyme A, all of which have a protective effect against cell damage. Dexpanthenol, which has anti-oxidative and anti-inflammatory effects, can be used both topically and systemically. Systemic Dexpanthenol is used in clinical practice primarily for its beneficial effects on postoperative enteroparesis and burning feet syndrome.
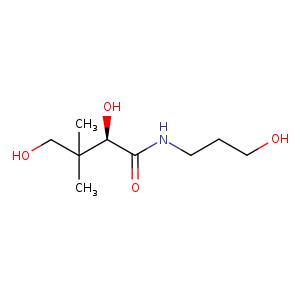
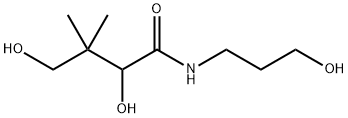
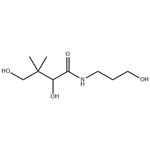
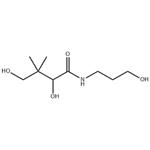
Figure 1. The molecular formula of Dexpanthenol
Application
Dexpanthenol is a widely used constituent of several ointments, eyedrops, injections and tablets. Its Beneficial effects have been observed in patients who have undergone skin transplantation or scar treatment, or therapy for burn injuries and different dermatoses. The stimulation of epithelization, granulation and mitigation of itching were the most prominent effects of formulations containing dexpanthenol[2]. Dexpanthenol is well absorbed when applied topically to the skin and rapidly converted to pantothenic acid. The latter is a coenzyme A constituent and essential for the physiological function of epithelia. It supports skin regeneration by enhancing epidermal differentiation and facilitates wound healing; it also showed activity in the prevention of biofilm formation and has anti-inflammatory effects. Furthermore, it acts as a moisturizer and skin barrier enhancer. In dry skin conditions, it compensates for reduced hydration by increasing water content and by beneficially influencing the molecular mobility of the stratum corneum lipid lamellae and proteins. These features triggered the development of various topical dexpanthenol-containing galenical formulations which are widely used in the dermatological field. Topical dexpanthenol has also been recommended for the treatment of minor and superficial wounds.
Safety and Pharmacology
Although isolated reports of itching, tingling, difficulty in breathing erythema, generalized dermatitis, urticaria, temporary respiratory difficulty (when dexpanthenol injection was admin 5 minutes after succinylcholine had been discontinued), hypotension, persistent (up to 10 days) diarrhea, & agitation have been assoc with use of dexpanthenol injection, a causal relationship to the drug has not been established.
Dexpanthenol injection should not be used for the management of mechanical obstruction; in these patients, therapy should be directed mainly at correcting the obstruction. The manufacturer of dexpanthenol injection cautions that the management of adynamic ileus includes correction of fluid & electrolyte abnormalities (especially hypokalemia), anemia, and hypoproteinemia; treatment of infection; avoidance of drugs that decrease GI motility; and decompression of the GI tract using nasogastric suction or a Iong intestinal tube when there is considerable distention.
The manufacturers state that dexpanthenol may prolong bleeding time, therefore, should be used with extreme caution, if at all, in hemophiliacs. It should be discontinued if signs of hypersensitivity occur during use.
Animal reproduction studies have not been performed with dexpanthenol. It is not known whether dexpanthenol can cause fetal harm when admin to pregnant women. Dexpanthenol injection should be used during pregnancy only when clearly needed. Since it is not known whether dexpanthenol is distributed into milk, dexpanthenol injection should be used with caution in nursing women[3].
It has been reported that dexpanthenol prolonged the muscle relaxant effects of succinylcholine; however, a controlled trial failed to show this effect. The manufacturers recommend that dexpanthenol not be administered within 1 hour of succinylcholine.
On theoretical grounds, the manufacturers of dexpanthenol recommend that the drug not be given with or within 12 hours after administration of neostigmine or other parasympathomimetic drugs. Although the clinical importance has not been established, the miotic effects of anticholinesterase ophthalmic preparations (e.g., echothiophate iodide (no longer commercially available in the US), isoflurophate) reportedly may be potentiated by pantothenic acid[4].
References
1.Roberts H , Williams J , Tate B . Allergic contact dermatitis to panthenol and cocamidopropyl PG dimonium chloride phosphate in a facial hydrating lotion[J]. Contact Dermatitis, 1977, 1977(6):591-592.
2.Ebner, F., Heller, A., Rippke, F. et al. Topical Use of Dexpanthenol in Skin Disorders. Am J Clin Dermatol , 2002(3):427–433.
3.McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2000.Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2000 (Plus Supplements)., p. 3317.
4.American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009).
Related articles And Qustion
Lastest Price from Panthenol manufacturers
US $0.00/kg2025-11-19
- CAS:
- 16485-10-2
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- Customise
US $0.00-0.00/kg2025-08-21
- CAS:
- 16485-10-2
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 1