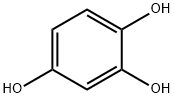
The Application of 1,2,4-Benzenetriol
1,2,4-Benzenetriol is a benzene ring compound bearing three hydroxyl functional groups. This trihydroxybenzene exhibits high chemical reactivity and serves as a metabolite of benzene in vivo. Functioning as a versatile intermediate, 1,2,4-benzenetriol finds extensive applications in the synthesis of derivatives for dyes, polymer materials, and related fields.
The hematotoxicity of 1,2,4-benzenetriol
The hematotoxicity of benzene is not attributed to benzene itself, but rather to its metabolic products in vivo, such as phenol, hydroquinone, catechol, and 1,2,4-benzenetriol. The benzene metabolite 1,2,4-benzenetriol exhibits dose-dependent cytotoxic effects on K562 cells within a specific concentration range. This cytotoxicity is likely mediated through alterations in the cell cycle, leading to the induction of apoptosis. Notably, even at non-cytotoxic low concentrations, 1,2,4-benzenetriol significantly inhibits hemin-induced erythroid differentiation in K562 cells. These dual effects—apoptosis induction and differentiation inhibition—may play significant roles in the mechanisms underlying benzene-induced hematotoxicity.[1]
The Application of 1,2,4-benzenetriol
Bio-based 1,2,4-benzenetriol(BTO), an aromatic product attainable from 5-hydroxymethylfurfural (HMF), can be used as a polyphenolic building block. Ciaran Lahive and co-worker shown that BTO possesses interesting reactivity. it was shown that this reactivity can lead to formation of bio-based compounds that can have potential as chemical intermediates. Firstly, it was shown that 5-5'-linked BTO dimer [1,1'-biphenyl]-2,2',4,4',5,5'-hexaol is formed exclusively under aerobic conditions at room temperature, which likely proceeds via a radical intermediate. Furthermore, BTO can be subsequently oxidized to afford a hydroquinone-containing dimeric structure, 2',4,4',5'-tetrahydroxy-[1,1'-biphenyl]-2,5-dione, under the same conditions. It was also demonstrated that dimer BTO, as well as a condensed dimer dibenzo[b,d]furan-2,3,7,8-tetraol, can be readily produced from BTO. The symmetric nature of these two dimeric products provide a potential platform for bio-based polymeric products, as for example bisphenol A18 replacements. Additionally, it was shown that the specific sequential deuterium exchange of the aromatic C-H's of BTO is readily achieved under acidic conditions at room temperature. Deuterium incorporation first occurs at the 5 position followed by the 3 position to yield BTO with 4 and 5 deuterium atoms respectively, which is likely to proceed through electrophilic aromatic substitution. These studies exemplify two types of reactivity that BTO can be involved in, which should be considered when working with this compound and which can also be exploited for accessing bio-based compounds from BTO.[2]

Fig. formation of BTO and BTO chemistry
Typical examples of a laminated optical disk include DVD (digital versatile disk or digital video disk). This DVD is produced by a method of at least laminating two substrates for optical disk, the substrate being obtained by forming an information recording layer on one substrate for optical disk. A laminating agent used in case of lamination is generally an adhesive comprising an ultraviolet curable composition. 1,2,4-benzenetriol used as a basic composition in adhesive.[3]
1,2,4-benzenetriol is the start material of hair dyeing compositions. It is known practice to obtain "permanent" colorations with dyecompositions containing oxidation dye precursors, which are generallyknown as oxidation bases, such a s ortho- or para-phenylenediamines,ortho- or para-aminophenols and heterocyclic compounds. These oxidation bases are colourless or weakly coloured compounds, which,2 0 when combined with oxidizing products, may give rise to coloured compounds by a process of oxidative condensation. It is also known that the shades obtained with these oxidation bases may be varied by combining them with couplers or coloration modifiers, the latter being chosen especially from aromatic meta-diamines, meta-aminophenols,2 5 meta-diphenols and certain heterocyclic compounds such as indole compounds.[4]
Plasticisers are colourless, odourless, organicchemicals used to soften polyvinyl chloride (PVC) and other polymers creating a whole new world of soft and bendable polymers for high performing applications and uses, which bring a myriad of benefits to everyday life. Tri-esters of 1,2,4-benzenetriol demonstrate good resistance to high temperatures with retention of the mechanical properties. They are also very cheap in production.[5]
References
[1]Xue, Ming, Effect of benzene metabolite 1,2,4-benzenetriol on proliferation and erythroid differentiation in K562 cells, [J] Dulixue Zazhi (2009), 23(6), 425-428
[2]Ciaran Lahive, Bio-based chemicals: 1,2,4-benzenetriol, selective deuteration and dimerization to bifunctional aromatic compounds, [J] Organic Process Research & Development (2018), 22(12), 1663-1671
[3]Dainippon Ink and Chemicals, Inc. ,Optical disk having specific hydroxyphenol derivative and silver-based reflective layer, [P] EP1669989
[4]Rue Royale, Hair dyeing compositions comprising an o-diphenol, a manganese or zinc salt, hydrogen peroxide, carbonate, and a titanium or scandium salt, [P]WO2012175683
[5]Avraham Meshulam, Tri-esters of 1,2,4-benzenetriol as plasticisers in the polymeric compositions, [P] US20180340054
You may like
Lastest Price from 1,2,4-Benzenetriol manufacturers
US $1.10-9.90/KG2025-08-20
- CAS:
- 533-73-3
- Min. Order:
- 1KG
- Purity:
- 99%min
- Supply Ability:
- 100KG

US $300.00-210.00/KG2025-04-29
- CAS:
- 533-73-3
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 2000KG