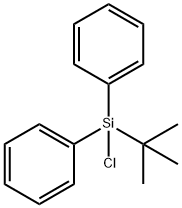
tert-Butylchlorodiphenylsilane: A Versatile Organosilicon Compound for Chemical Applications
Introduction to Tert Butylchlorodiphenylsilane
Tert Butylchlorodiphenylsilane, a silane protectant, is an organic silicon compound. Its function is to replace active hydrogen (such as hydrogen in hydroxyl, carboxyl, and amino groups) in silane-based compounds, generating stable intermediates; Then, other functional groups of the intermediate undergo certain reactions; After the reaction is completed, the silane group is removed through hydrolysis reaction to regenerate the groups originally protected by the silane group, and certain specific compounds are synthesized. Due to the high conversion rate and even quantitative reaction of silane protection and deprotection reactions, it is widely used in organic synthesis, especially in drug synthesis.

Figure 1 Characteristics of Tert Butylchlorodiphenylsilane
Partial properties of Tert Butylchlorodiphenylsilane
1. The appearance of Tert Butylchlorodiphenylsilane is a colorless liquid.
2.. High purity Tert Butylchlorodiphenylsilane is stable in water and air.
The main uses of Tert Butylchlorodiphenylsilane
Tert Butylchlorodiphenylsilane is used as a reagent in silicon compounds and organic synthesis. It can be used for synthesizing organosilicon compounds, such as organosilicon polymers and silicone grease. TBPCl is also suitable as a precursor for catalysts and in other organic synthesis reactions.
Preparation of Tert Butylchlorodiphenylsilane
1. The method for preparing Tert Butylchlorodiphenylsilane is usually obtained by reacting hydrocarbons (such as phenyl) with chlorosilanes (such as trichlorosilane). This reaction requires appropriate reaction conditions and catalysts.
2. Add 200ml of tetrahydrofuran, 12g of magnesium, and 6g of chloropropene (the remaining 40g of chloropropene is added to the dropping funnel) into a 500Ml reaction kettle with a snake shaped condenser, electric stirrer, dropping funnel, thermometer, and heater. Slowly heat the kettle while stirring to initiate the reaction, controlling the reaction temperature at 50-75 ℃. Once the reaction is initiated, stop heating immediately. If the reaction is severe, quickly cool the reaction kettle. After the reaction is stable, open the dropping funnel and drop chloropropene into the reaction kettle. After the addition is complete, lower it to room temperature. Then add 0.2g of sodium thiocyanate and 0.2g of cuprous chloride catalyst to the reaction kettle. Add 127g of diphenyl dichlorosilane to the dropping funnel while stirring. Add diphenyldichlorosilane dropwise into the reaction vessel. After the dropwise addition is complete, heat the material in the reaction vessel to 90-150 ℃ and maintain a constant temperature for 4-7 hours. The reaction is complete, After cooling to room temperature, add 100mL of inert solvent toluene to the reaction kettle, stir evenly, filter the resulting liquid to remove solid salts, distill the filtrate to remove the flux, and then perform vacuum distillation. At 0.09Kpa, collect the fraction at 200 ℃ to obtain Tert Butylchlorodiphenylsilane, a product with a content of 98%.
Application of Tert Butylchlorodiphenylsilane
pharmaceutical intermediates
Tert Butylchlorodiphenylsilane is widely used as a pharmaceutical intermediate. It plays a crucial role in the process of drug synthesis, helping to construct complex organic molecular structures. For example, in the synthetic pathway of certain drugs, it can be used as a protective group to protect hydroxyl or other sensory groups from unnecessary side reactions in subsequent reactions.
Silicification agent
As a silicifying agent, Tert Butylchlorodiphenylsilane can be used to protect alcohols and other sensory groups. This protective strategy is very common in organic synthesis, especially in multi-step synthesis processes, where certain functional groups need to be temporarily masked to avoid their involvement in unwanted reactions. In the final step, these protective groups can be removed under appropriate conditions to restore the original functional groups.
Precautions for Tert Butylchlorodiphenylsilane
1. Tert Butylchlorodiphenylsilane is an organic compound that should be avoided from contact with the skin, inhalation of vapors, or ingestion.
2. During the use of Tert Butylchlorodiphenylsilane, personal protective equipment must be worn, including lab coats, chemical gloves, and protective goggles.
3. When using and storing Tert Butylchlorodiphenylsilane, sources of ignition should be avoided as Tert Butylchlorodiphenylsilane is flammable.
4. When handling Tert Butylchlorodiphenylsilane, it is necessary to operate in a well ventilated laboratory environment.
![Article illustration]() Reference
Reference
[1] Sluggett G W, Leigh W J. Photochemistry of 1, 2-di-tert-butyl-1, 1, 2, 2-tetraphenyl disilane, a clean, direct source of arylalkylsilyl radicals[J]. Organometallics, 1992, 11(11): 3731-3736.
[2] Deller K, Rieger B. Synthesis and characterization of variously asymmetrically chloro-substituted disilanes and trisilanes–A new perspective on known compounds[J]. Journal of Organometallic Chemistry, 2015, 794: 188-196.
You may like
Related articles And Qustion
See also
Lastest Price from tert-Butylchlorodiphenylsilane manufacturers

US $0.00/kg2025-09-24
- CAS:
- 58479-61-1
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 1000kgs

US $0.00/KG2025-04-21
- CAS:
- 58479-61-1
- Min. Order:
- 1KG
- Purity:
- 98%min
- Supply Ability:
- 30tons/month