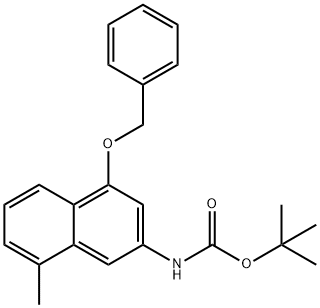
tert-butyl (4-(benzyloxy)-8-methylnaphthalen-2-yl)carbamate - Reaction / Application on Synthetic Works
Tert-butyl (4-(benzyloxy)-8-methylnaphthalen-2-yl)carbamate is an important organic intermediate (building block) to synthetize substituted methylnaphthalen products.
The following example is about its application on the synthesis of duocarmycin prodrugs[1].

Tert-butyl (4-(benzyloxy)-8-methylnaphthalen-2-yl)carbamate (10.5 g) was treated with 1.01 equivalents of N-bromosuccinimide (5.20 g) in THF (31.5 g) at -10 °C. The reaction was quenched by addition of an aqueous sodium hydroxide solution (0.75 g NaOH and 7.5 g water). The extract was washed with a saturated aqueous NaC1 solution (15 g) and the water layer was extracted with ethyl acetate (11.25 g). The organic layers were combined, washed with a saturated aqueous NaC1 solution (15 g) and concentrated under vacuum to yield tert-butyl (4- (benzyloxy)- 1 -bromo- 8-methylnaphthalen-2-yl)carbamate (14.36 g, 112.36 percent yield).
The following example is about its application on the synthesis of linker-duocarmycin payloads[2].

Tert-butyl (4-(benzyloxy)-8-methylnaphthalen-2-yl)carbamate (103.4 g, 207 mmol) was dissolved in THF (1 L). The solution was cooled to −25 °C under an atmosphere of nitrogen. Then n-BuLi (100 mL, 2.5 M in hexanes) was added gradually, while the temperature was kept at −25 to −20 °C. The mixture was stirred for an additional 10 min and then quenched with a saturated aqueous NH4Cl solution. The mixture was extracted twice with EtOAc (2 × 500 mL). An aqueous solution of p-toluenesulfonic acid (12 g of monohydrate in 50 mL of water) was added to the combined organic layers, and the reaction mixture was stirred for 1 h. The reaction was quenched by addition of a 1 M aqueous Na2CO3 solution. Layers were separated and the organic layer was washed with saturated aqueous NaCl, dried with MgSO4, and concentrated.
Crude product was dissolved in DCM and filtered over silica gel (0.063−0.1 mm, 60 Å). Elution was carried out with DCM (2.2 L) followed by DCM/EtOAc (2.7 L, 9:1, v/v) and DCM/EtOAc (2.4 L, 4:1, v/v). The fractions were combined, the resultant solution was concentrated and dried, and the residue was crystallized from DCM/pentane. Crystals were collected and dried to give the product (34.7 g, 40%) as a beige to white solid.
The following example is about its application on the synthesis of the building block of the binding molecule of SYD985 [2].

Tert-butyl (4-(benzyloxy)-8-methylnaphthalen-2-yl)carbamate (17.0 g, 40.5 mmol) was treated with methane sulfonyl chloride (4.08 mL, 52.7 mmol) and Et3N (14.6 mL, 105 mmol) in CH2Cl2 (140 mL) for 90 min at 0−5 °C. The reaction mixture was washed with hydrochloric acid, water, and saturated aqueous NaCl, dried with MgSO4, and concentrated. The residue, comprising the intermediate mesylate, was dissolved in DMF (120 mL), and the solution was treated with LiCl (8.59 g, 203 mmol) at 80 °C for 90 min. After evaporation of DMF, the residue was partitioned between CH2Cl2 and water. The layers were separated, and the organic layer was washed with saturated aqueous NaCl, dried with MgSO4, and concentrated. The residue was dissolved in hot heptane (350 mL), and the mixture was treated with activated carbon and filtered. The activated carbon was washed with another portion of heptane. The combined filtrate was cooled to approximately 50 °C, seeded, and kept at this temperature for 1 h. The suspension was cooled to 7 °C over 2 h and stirred for another hour at this temperature. Crystals were filtered, washed with heptane, collected, and dried. Dried crystals were recrystallized from heptane using the procedure described above, giving the product (11.1 g, 63%; enantiomeric excess,
99.99%)
References
1.Synthon Biopharmaceuticals B.V. Huijbrgts T, Elgersma RC, Beusker PH, Joosten JAF, Coumans GE, Spijker HJ, Menge W De Groot, Franciscus MH. Improved process for making duocarmycin prodrugs. WO2015/185142[P], 2015, A1, Page column 13, 14, 15, 16.
2.Elgersma RC, Coumans RGE, Huijbregts T, Menge WMPB, Joosten JAF, Spijker HJ De Groot, Franciscus MH, Timmers CMarco, Beusker PH. Design, synthesis, and evaluation of linker-duocarmycin payloads: Toward selection of HER2-targeting antibody-drug conjugate SYD985[J]. Molecular Pharmaceutics, 2015, 12(6):1813-1835

![945864-47-1 (S)-3-Boc-5-(benzyloxy)-1-(chloromethyl)-9-methyl-2,3-dihydro-1H-benzo[e]indole; reaction; uses](httpss://www.chemicalbook.com/NewsImg/2019-11-29/201911291029456958.jpg)