Systemic effects of Sevoflurane
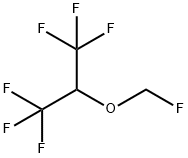
Sevoflurane is a polyfluorinated isopropyl methyl ether (fluoromethyl-2,2,2-trifluoro-1-ethyl ether). Unlike the other volatile agents, sevoflurane is achiral.
Uses
1. Induction and maintenance of general anaesthesia. 2. Sedation on ITU. 3. Treatment of severe asthma in patients whose lungs are mechanically ventilated.
Physical properties
Sevoflurane is non-flammable and has a pleasant smell. It has a low blood/gas partition coefficient close to those of desflurane and nitrous oxide. During storage, where the concentration of added water is less than 100ppm, it is susceptible to attack by Lewis acids (defined as any substance that can accept an electron pair) resulting in the release of hydrofluoric acid, which is highly toxic. This can be compounded if sevoflurane is stored in glass bottles as the hydrofluoric acid can corrode glass, formulating further Lewis acids. Consequently, sevoflurane is formulated with 300ppm water and stored in polyethylene naphtholate or epoxy phenolic resin–lined aluminium bottles to ensure stability.
Systemic effects
RS:
• Non-irritant to the upper respiratory tract; suitable for gaseous induction.
• Dose-dependent depression in tidal volume.
• Reduced respiratory drive in response to hypoxaemia.
• Reduced respiratory drive in response to raised PaCO2 to a similar degree to other volatile agents.
• Increased respiratory rate.
• Inhibits hypoxic pulmonary vasoconstriction.
• Bronchial smooth muscle relaxation.
CVS:
• Increased HR (less than that of isoflurane).
• Not associated with coronary steal.
• Dose-related reduction in SVR, myocardial contractility and MAP.
• Does not sensitise the myocardium to exogenous catecholamines.
CNS:
• Causes general anaesthesia.
• Dose-dependent reduction in cerebral vascular resistance and cerebral metabolic rate.
• Preferred volatile agent for neuroanaesthesia. Although ICP is increased at high inspired concentrations (> 1.5 MAC) this effect is minimal over the 0.5–1.0 MAC range. This is thought to be due to preserved cerebral autoregulation of CBF.
• No excitatory effects on EEG.
• Potentiation of NMBAs.
• Dose-dependent muscle relaxation.
GI/GU:
• No demonstrable renal toxicity clinically despite concerns of fluoride ion exposure.
• Preservation of renal blood flow.
• Uterine relaxation.
• Increased risk of PONV.
Other:
• Trigger for MH.
• Production of potentially toxic compounds when in prolonged contact with soda lime (see next section).
Pharmacology
Uptake:
• Rapid onset/offset because of a low blood/gas partition coefficient.
Metabolism:
• Approximately 2%–3% of the absorbed dose is metabolised (by defluorination) in the liver (CYP450 2E1) to HFIP, carbon dioxide and inorganic fluoride. Inorganic fluoride concentrations peak within 2 h of the end of anaesthesia and have a half-life of 15–23 h. There have been no reports of fluoride toxicity in clinical studies investigating sevoflurane.
Excretion:
• Approximately 98% is excreted unchanged from the lungs. Hexafluoroisopropanol is conjugated with glucuronic acid as excreted as urinary metabolite.
See also
Lastest Price from Sevoflurane manufacturers

US $0.00-0.00/KG2025-05-30
- CAS:
- 28523-86-6
- Min. Order:
- 1KG
- Purity:
- 98.0%
- Supply Ability:
- 10000KGS

US $0.00-0.00/kg2024-11-01
- CAS:
- 28523-86-6
- Min. Order:
- 1kg
- Purity:
- USP
- Supply Ability:
- 100kg