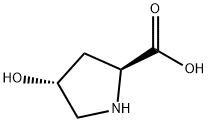
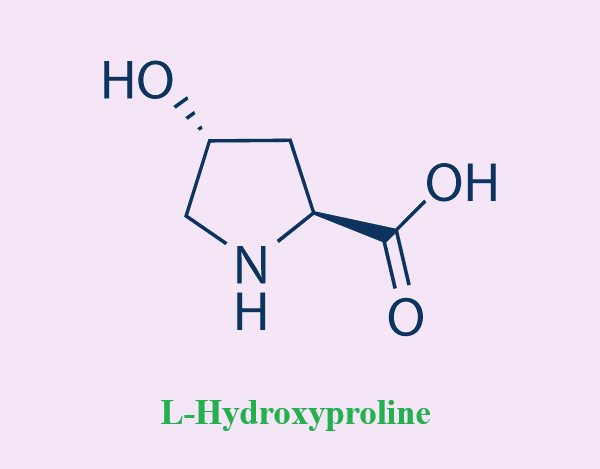
Synthesize and physical properties of L-hydroxyproline
The solubility of l-hydroxyproline
Solubility and phase diagrams play an important role in pharmaceutical crystallization, and it guide the crystallization process, such as choice of pure solvent or mixtures, cooling, evaporation or anti-solvent crystallization. Solubility and dissolving rate also determine the drug release and drug delivery. Solubility and dissolving rate also determine the drug release and drug delivery[1]. Solubility of l-hydroxyproline in water and binary solvent mixtures with methanol, ethanol or n-propanol was systematically determined in a temperature range of 293.15 K–318.15 K under 0.1 MPa. The solubility increased with temperature and increased with increasing composition of water in the binary solvent mixtures.
The specific determination of L-hydroxyproline (Hyp)
The specific determination of L-hydroxyproline (Hyp) can serve as a potential indicator for early clinical diagnosis of liver fibrosis[2]. Liver fibrosis is one of the major causes of morbidity and mortality worldwide. The progression of liver fibrosis often develops into irreversible cirrhosis or even liver cancer. In order to avoid developing into terminal illness, researches on molecular metabolic biomarker in fluid or tissue of liver fibrosis are crucial. In the past decade, metabolomics has become a novel method in the identification and/or quantitation of small molecules or metabolites for diseases because it can comprehensively characterize small molecule metabolites in biological systems and provide an overview of the metabolic status. L-hydroxyproline (Hyp) contents change can provide valuable information about biochemical and pathologic events and assess the degree of liver fibrosis. The development of sensitive, accurate, rapid and specific targeted analytical method for Hyp detection has an important significance for the early clinical diagnosis of liver fibrosis.
The L-hydroxyprolines (L-Hyp) synthesize
Post-translational hydroxylation of the L-proline residue mainly occurs in collagen; therefore, the L-hydroxyprolines (L-Hyp) synthesized, including trans-4-hydroxy-L-proline (T4LHyp) and trans-3-hydroxy-L-proline (T3LHyp), are important markers for directly measuring the content of collagen in several biological samples. The most frequently used method to estimate the content of L-Hyp is high-performance liquid chromatography (HPLC), which is inconvenient. In the present study, we attempted to estimate the content of L-Hyp using coupling systems with metabolic enzymes of the T4LHyp (hydroxyproline 2-epimerase (HypE) and cis-4-hydroxy-D-proline dehydrogenase (HypDH)) and T3LHyp pathways (T3LHyp dehydratase (T3LHypD) and Δ1-pyrroline-2-carboxylate reductase (Pyr2CR)) from microorganisms. We constructed a functional expression system of recombinant HypDH with a heterooligomeric structure in Escherichia coli cells. Enzymological characterization revealed that the β-subunit acted as a catalytic subunit, and also that assembly with other subunit(s) improved the kinetics for cis-4-hydroxy-D-proline and thermostability. By using a spectrophotometric assay with different wavelengths, the contents of T4LHyp and T3LHyp were successfully estimated within the ranges of 0.004–1 mM and 0.05–1 mM, respectively, and were consistent with those determined by HPLC. This enzymatic method was used to measure the content of T4LHyp in the acid-hydrolysate of collagen, and blood plasma[3].

Picture 1 L-Hydroxyproline powders
An endogenous precursor of oxalate
As calcium oxalate is the most common constituent of urinary stones animal model of the urolithiasis has been established by feeding L-hydroxyproline, an endogenous precursor of oxalate. However, the influence of oral L-hydroxyproline on human urinary oxalate excretion has not been clearly demonstrated[4]. We herein report a clinical study examining the influence of daily ingestion of L-hydroxyproline on urinary oxalate levels with a focus on measuring oxalic acid excreted in the urine. there are various opinions on the association of dietary and urinary oxalate. Recently, it was reported that the Dietary-Approaches to Stop Hypertension (DASH)-style diet is more effective in reducing calcium oxalate supersaturation than a low-oxalate diet, although it includes a lot of fruits and vegetables which contain much oxalate [4]. Thus, the influence of the diet in conjunction with oxalate is still unclear in human metabolism. Moreover, we found no accumulation of L-hydroxyproline and oxalate in the withdrawal period same as the animal model. The metabolic significance of L-hydroxyproline is still unclear and further investigation is warranted.
Reference
1 Zhu, Yitong; Yang, Huaiyu; Si, Zehao; Zhang, Xiangyang (2019). Solubility and thermodynamics of l-hydroxyproline in water and (methanol, ethanol, n-propanol) binary solvent mixtures. Journal of Molecular Liquids, 112043
2 Zhu, Shuyun; Zheng, Longfang; Sun, Luping; Jia, Wenhui; Sun, Jing; Zhao, Xian-En; Liu, Huwei (2020). Derivatization-based magnetic dummy molecularly imprinted polymers integrated with 4-plex stable isotope labeling derivatization strategy for specific and rapid determination of L-hydroxyproline in human serum. Analytica Chimica Acta, 1127(), 57–68.
3 Takayama T, Fujita K, Suzuki K, et al. Control of oxalate formation from L-hydroxyproline in liver mitochondria[J]. Journal of the American Society of Nephrology, 2003, 14(4): 939-946.
4 Watanabe S, Hiraoka Y, Endo S, et al. An enzymatic method to estimate the content of L-hydroxyproline[J]. Journal of Biotechnology, 2015, 199: 9-16.
Related articles And Qustion
See also
Lastest Price from L-Hydroxyproline manufacturers

US $1.00-4.00/KG2025-05-19
- CAS:
- 51-35-4
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 200000KG

US $5.00-0.50/KG2025-05-08
- CAS:
- 51-35-4
- Min. Order:
- 1KG
- Purity:
- 99% hplc
- Supply Ability:
- 500TONS