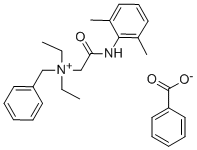
Synthesis, Toxicity and Detection Method of Denatonium Benzoate
General description
Denatonium Benzoate is the bitterest compound known to exist in the world. If you set the bitterness level of quinine as baseline 1, this product has a bitterness level of 1000 and a concentration of 10 ppm. For most people, this product is unbearably bitter and most of its applications are related to its bitterness. It is a cheap and efficient substitute for strychnine, equitrin, quinine, lignin and naringin. Denatonium Benzoate (bitter) is currently used as an aversive agent, denaturing agent, food blocking agent and flavoring agent. Bitters are included as inactive ingredients in the United States Pharmacopoeia USP32?NF27.
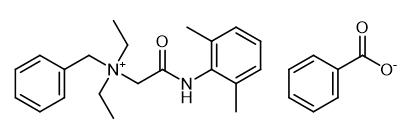
Fig. 1 The structure of Denatonium Benzoate.
Denatonium Benzoate is a quaternary ammonium salt formed by the combination of a quaternary ammonium cation with an inert anion such as benzoate ion or saccharin anion. The cation is structurally similar to the local anesthetic lidocaine except that a benzyl functional group is added to the nitrogen atom of the amino group.
Physicochemical property
Denatonium Benzoate is a white to off-white solid with a melting point of 164-168 °C (lit.). Its boiling point and density are approximately 555.91°C and 1.1256. It is generally stable, but it is incompatible with strong oxidizing agents.
Synthetic routes
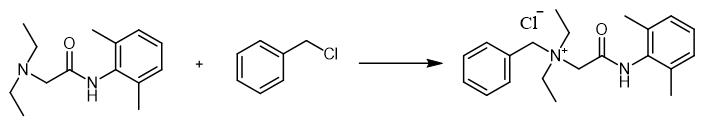
Fig. 2 The synthetic step 1 of Denatonium Benzoate.
Introduce 3.36 g (14.34 mmol) of lidocaine, 2 cm3 acetonitrile, 1.88 g (14.85 mmol) of benzyl chloride and 34 mg (0.2 mmol) of potassium iodide into the round-bottom flask. Then mix and heat the content of flask under reflux at 80 ℃. After 2 hours remove the acetonitrile under reduced pressure on a rotary evaporator. In next step crush the raw product to pieces with spatula and mix it with 20 cm3 of ethyl acetate. Then, separate the obtained mixture using vacuum filtration and wash the solid with second portion (10 cm3) of ethyl acetate. Later dry the crude product under vacuum for 1 hour at 80 ℃ [1].

Fig. 3 The synthetic step 2 of Denatonium Benzoate.
Introduce 1.30 g (3.6 mmol) of denatonium chloride, 5 cm3 of commercial ethanol with 0.20 g (3.6 mmol) of potassium hydroxide to the round-bottom flask. Mix the content of the flask for 10 minutes and then cool it to the temperature of 4℃. Then, separate the precipitated potassium chloride via vacuum filtration, wash the sediment with 2 cm3 of cold ethanol [1].
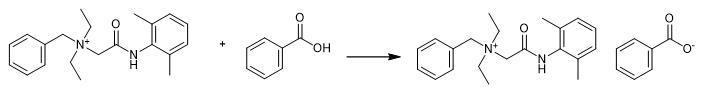
Fig. 4 The synthetic step 3 of Denatonium Benzoate.
Subsequently, neutralize filtrate with 0.44 g (3.6 mmol) of benzoic acid (Note: Water-moistened test strip should be used to detect the end of neutralization). In the next step, evaporate the solvent from solution under reduced pressure in the rotary evaporator. As a result, 1.55 g (96%) of white solid, characterized by melting point in a range from 164.0 to 168.0 ℃ should be obtained [1].
Toxicity and Efficacy
When ingested at 10 ppm by human beings, denatonium benzoate has an extremely bitter, unpleasant taste. The addition of denatonium benzoate to liquid dish detergents and orange juice reduces the amount ingested by children. The toxicity of denatonium benzoate is low with acute po LD50's in rats of 485-740 mg/kg. The use of bittering agents, such as denatonium benzoate, could reduce the ingestion of toxic substances by dogs, Cats [2].
Experiments were conducted to determine the concentration of denatonium benzoate at which feed becomes unpalatable to captive red deer (Cervus elaphus) and roe deer (Capreolus capreolus) to identify the concentrations likely to be required to prevent trees being browsed by deer. When offered in a mixture of barley and straw as a single feed, red deer showed no reduction in feed intake with denatonium benzoate concentrations up to 1000 ppm. When offered a choice of two feeds, one containing denatonium benzoate, both red and roe deer showed a preference for the denatonium benzoate-free feed. The results indicate that deer avoid feed containing denatonium benzoate and thus the chemical could potentially prevent the browsing of trees by deer [3].
Research was conducted among young children to determine whether or not the bitter tasting substance denatonium benzoate added to liquid detergents would effectively reduce the risk that large quantities of such products might be ingested. The research design involved essentially no risk to the young subjects and was approved by an Institutional Review Board prior to initiation. The value of an effective deterrent material is apparent based on the number of reported ingestions of detergent products each year. Denatonium benzoate was chosen as a potential deterrent based on its existing uses in alcohol as a denaturant and in thumb-sucking and nail-biting deterrent products. In the present research, 108 children ranging in age from 18 to 47 months were offered a drink of either a dilute soapy tasting solution or that same solution with denatonium benzoate added. The results showed that for the liquid containing denatonium benzoate, significantly less liquid was ingested, significantly fewer subjects accepted a second taste, and significantly more subjects displayed behavior indicating immediate and intense aversion to the taste of denatonium benzoate. The data indicate that the addition of denatonium benzoate to a liquid detergent would be expected to significantly reduce, but most likely not eliminate, the probability of an accidental ingestion involving multiple swallows by a young child [4].
Detection method
A poly(vinyl chloride) matrix membrane ion-selective electrode for the determination of the denatonium ion based on the denatonium salt of tetraphenylborate is described. The response characteristics of the electrode for the denatonium ion and for several quaternary ammonium compounds were studied. The potentiometric determination of denatonium benzoate in rapeseed oil in the range 1-10 ppm agreed to within +/- 5% of the spiked amounts [5].
References
[1] Stachowiak W, Wysocki M, Niemczak M. “Bitter” Results: Toward Sustainable Synthesis of the Most Bitter Substances, Denatonium Saccharinate and Denatonium Benzoate, Starting from a Popular Anesthetic, Lidocaine[J]. Journal of Chemical Education, 2022, 99(4): 1604-1611.
[2] Hansen S R, Janssen C, Beasley V R. Denatonium benzoate as a deterrent to ingestion of toxic substances: toxicity and efficacy[J]. Veterinary and human toxicology, 1993, 35(3): 234-236.
[3] Wright I A, Milne J A. Aversion of red deer and roe deer to denatonium benzoate in the diet[J]. Forestry: An International Journal of Forest Research, 1996, 69(1): 1-4.
[4] Berning C K, Griffith J F, Wild J E. Research on the effectiveness of denatonium benzoate as a deterrent to liquid detergent ingestion by children[J]. Fundamental and Applied Toxicology, 1982, 2(1): 44-48.
[5] Nambiar O G B, Gosavi K, Ravindranathan T. Plastic membrane ion-selective electrode for the determination of denatonium benzoate (Bitrex)[J]. Analyst, 1991, 116(10): 1011-1012.
Related articles And Qustion
See also
Lastest Price from Denatonium Benzoate manufacturers
US $1.00-4.00/KG2025-09-02
- CAS:
- 3734-33-6
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 200000KG

US $0.00-0.00/Kg2025-04-21
- CAS:
- 3734-33-6
- Min. Order:
- 1Kg
- Purity:
- 98%
- Supply Ability:
- 20Ton