Synthesis technology and solubility of 1,1,1-Tris(hydroxymethyl)ethane
Introduction
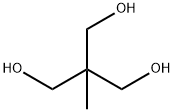
1,1,1-Tris(hydroxymethyl)ethane (Figure 1) is a kind of important chemical intermediate and fine chemical products. It can react with organic acid generated monoester or polyester;with aldehydes,ketones generated acetal, ketal; with diisocyanate generated carbamic acid ester, etc. It is easy to manufacture, wide application, market demand is good.[1]
1,1,1-Tris(hydroxymethyl)ethane, an inexpensive and commercially available tripod ligand, has been used in the synthesis of some metal clusters for their magnetic studies. Results show that this tripodal ligand can change the spin ground states of the species due to the nature of its special skeleton. On the other hand, an interesting study has been reported that complexes of the type CuI(tripod)X [tripod=1,1,1-tris(diphenylphosphanyl)ethane; X-=halide, thiophenolate, phenylacetylide] are phosphorescent in solution and in the solid state. It is suggested that the emission is facilitated by the rigid tetrahedral structure, which is imposed by the tripod ligand. Nevertheless, no further reports dealing with these tripodal ligands in metal-catalyzed cross-coupling reactions have been presented to date. Finally, Buchwald and co-workers have highlighted that ethylene glycol can act as an effective bidentate O-donor ligand in the copper-catalyzed N- and S-arylation of aryl iodides with amines and thiols,respectively. Notably, they also pointed out that amides and anilines were poor substrates in their Cu-catalyzed protocol. On the basis of these studies, researchers were prompted to examine whether this tripodal ligand can be presented as a new class of tridentate O-donor ligands suitable to be used in copper-catalyzed cross-coupling reactions. Herein, researchers report that this inexpensive and commercially available triol,1,1,1-tris(hydroxymethyl)ethane, can be used as an efficient,versatile, and novel tripod ligand in the copper-catalyzedformation of C-N, C-O, and C-S bonds between aryl iodides and amides, phenols, and thiols, respectively.[2]

Synthesis technology of 1,1,1-Tris(hydroxymethyl)ethane
Report 1
Using parafomaldehyde and propionaldehyde as starting material ammonia as condensation catalyst NaOH as Cannizzaro reaction cataslyst 1,1,1-tris(hydroxymethyl)ethane is synthesized by two steps reaction The effects of different kind of catalysts including ammonia diethylamine and triethylamine the amount of ammonia, the reaction temperature and time ane investigated. The optimum conditions are 15g paraformaldehyde 11mL propionaldehyde (98%), 40mL deionized-distilled water; the pH value of the reaction solution is adjusted to 8.4 by adding ammonia;temperature of condensation reaction is 55℃,condensation reaction time is 3.0 hour After the condensation reaction; 5mL 50% NaOH solution is injected; the temperature of Cannizzaro reactin is 70℃, Cannizzaro reaction time is 1.5hours Under these conditions, the yield of 1,1,1-tris(hydroxymethyl)ethane is 95.2%.[3]
Report 2
The main purpose of 1,1,1-tris(hydroxymethyl)ethane synthesis is to improve the reaction conversion rate, the experiment is mainly to study the effect of various experimental conditions on reaction, so as to get the best experimental conditions. In addition, the separation and purification technology of reaction product plays a vital role to reduce the cost of production and improve product quality. Propionic aldehyde and formaldehyde as the raw material, through the aldol condensation reaction generated intermediate 2,2-dimethylolpropionaldehyde, through Cannizzario disproportionation reaction generated aim product 1,1,1-tris(hydroxymethyl)ethane. The optimum conditions were obtained by the experiments as follow: n(propionic aldehyde) : n (formaldehyde)= 1:3.5, dropwise add aqueous solution sodium hydroxide, and dropwise add temperature was 30℃, condensation reaction temperature was 30℃, condensation reaction time was 3h, n (propionic aldehyde):n(sodium hydroxide) =1:1.3, concentration sodium hydroxide was 50%, m(propionic aldehyde) : m(water) =1:8, Cannizzario reaction temperature was 65℃, Cannizzario reaction time was 2h, respectively. The yield of 1,1,1-tris(hydroxymethyl)ethane reached 88.21%.
The solubility of 1,1,1-tris(hydroxymethyl)ethane in methyl alcohol, ethyl alcohol, ethyl acetate and acetone were determined by equilibrium method. and the experimental data were correlated by polynomial empirical equation, the Apelblat equation and λh equation,respectively. The polynomial empirical equation and Apelblat equation could well describe the solubility data of 1,1,1-tris(hydroxymethyl)ethane. The enthalpy and entropy of dissolution of 1,1,1-tris(hydroxymethyl)ethane were estimated based on regression of the solubility data by utilizing the van’t Hoff equation.And the dissolving process was endothermic and entropy-driving.Through analysing the experimental data of solubilities, Liu choose methanol as solvent to separate 1,1,1-tris(hydroxymethyl)ethane with by-products. Determined the dosage of methanol through the experiment. and choose the ethyl acetate as solvent to refine 1,1,1-tris(hydroxymethyl)ethane, the optimum conditions were obtained by the experiments as follow: the dosage of solvent was 400mL, crystallization temperature was 20℃, the cooling rate°C was 0.2℃/min, a crystal. The yield of product reached 88.21% and the purity reached 98.32%. The quality of purified 1,1,1-tris(hydroxymethyl)ethane meet the quality of product requirements through characterization analysis.[1]
1,1,1-Tris(hydroxymethyl)ethane Solubility in Different Solvents
1,1,1-tris(hydroxymethyl)ethane is an important chemical intermediate and its solid-liquid equilibrium data is the basis for separation and purification. The solubility of 1,1,1-tris(hydroxymethyl)ethane in methanol, ethanol,n-propanol, isopropanol and dioxane were measured by an equilibrium method at 288.15~318.15 K. The results show that the solubility of 1,1,1-tris(hydroxymethyl)ethane in the above five solvents increases with the increase of temperature. 1,1,1-Tris(hydroxymethyl)ethane has the highest solubility in methanol and the lowest in dioxane. The modified Apelblat equation and the λh equation were selected to correlate the experimental data, and the results show that the calculated values agree well with the experimental data. The experimental solubility data and their correlation equation can provide essential data for the synthesis and purification of 1,1,1-tris(hydroxymethyl)ethane.[4]
References
1.Liu S.Research On Synthesis And purification Process of Trimethylolethane[D].Hebei University of Technology,2016.
2.Chen YJ, Chen HH. 1,1,1-tris(hydroxymethyl)ethane as a new, efficient, and versatile tripod ligand for copper-catalyzed cross-coupling reactions of aryl iodides with amides, thiols, and phenols. Org Lett. 2006;8(24):5609-5612. doi:10.1021/ol062339h
3.Wu ZG,et al. Study of synthesis technology of 1,1,1-Tris(hydroxymethyl)ethane[J].Shanxi Chemical Industry,2009,29(06):9-11.DOI:10.16525/j.cnki.cn14-1109/tq.2009.06.013.
4.Shao XZ,et al.Measurement and Correlation of Trimethylolethane Solubility in Different Solvents[J].Journal of Chemical Engineering of Chinese Universities,2016,30(04):967-970.
You may like
Lastest Price from 1,1,1-Tris(hydroxymethyl)ethane manufacturers

US $10.00/KG2025-04-21
- CAS:
- 77-85-0
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt

US $99.00-35.00/kg2025-04-15
- CAS:
- 77-85-0
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 20ton