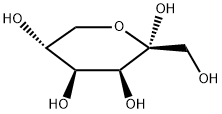
Synthesis of Tagatose
D-tagatose is a kind of rare sugar. It is a new functional sweetener discovered in recent years that has the functions of low-calorie, lowering blood sugar, regulating intestinal flora, and anti-caries. It was recognized as a generally recognized safe food by the US Food and Drug Administration in 2001, and was approved as a food raw material by my country's National Health and Family Planning Commission in 2014.

The sweetness of D-tagatose is similar to that of sucrose, the sweetness is 92% of that of sucrose, and there is basically no bad peculiar smell and aftertaste. The calories produced are only 1/3 of that of sucrose, and the energy value is 1.5kcal/g. Moreover, D-tagatose is prone to Maillard reaction, and caramelization reaction can occur at a relatively low temperature, so it has a wide range of application prospects in food fields such as dairy products, beverages, cereal products, candies, and preserved fruits. D-tagatose is a rare natural ketulose, which is an isomer of D-galactose, the epimer at the C-4 position of D-fructose.
Synthesis method
The natural D-tagatose is not easy to find, it is only found in the gum secreted by trees. At present, the production of D-tagatose mainly adopts chemical synthesis and biosynthesis.
1. Chemical synthesis
The chemical synthesis method uses soluble alkali metal or alkaline earth metal salt as a catalyst to catalyze the isomerization reaction of D-galactose and metal hydroxide under alkaline conditions to produce metal hydroxide and D-tagatose complex precipitation , And then add acid for neutralization and separation of precipitation to obtain the final product of D-tagatose. According to reports, Spherix of the United States, Arlafood of Denmark and Nutrilad of Belgium have applied chemical synthesis to industrial production. Compared with foreign countries, the chemical synthesis of D-tagatose in my country is still in the process of industrialization. Researchers are committed to improving the process conditions to increase the yield and purity of D-tagatose. At present, although chemical synthesis can achieve industrial production, it has certain shortcomings, such as many by-products and serious environmental pollution. Therefore, if chemical synthesis is to be further developed, further improvements must be made in the process.
2. Biosynthesis
The biosynthesis method uses biological enzymes or enzyme-containing cells as catalysts to catalyze the synthesis of D-tagatose from the substrate. Compared with chemical synthesis, it has the advantages of mild conditions, higher yield and fewer by-products. Currently, D-tagatose can be obtained by catalyzing the oxidation of galactitol by dehydrogenase, or D-tagatose can be generated by the isomerization of D-lactose catalyzed by L-arabinose isomerase.
2.1 Using galactitol as raw material to produce D-tagatose
Using galactitol as a raw material to produce D-tagatose is one of the earliest biosynthetic methods studied. In 1984, Izumori K et al. first used saponins and Arthrobacter globularis strains to oxidize galactitol to tagatose. Muniruzzaman S et al. found that the strain Enterobacter agglomerans isolated from the soil can oxidize galactitol to D-tagatose. The experimental results show that when the concentration of galactitol is 2%, the conversion rate is 92%, and when the concentration of galactitol is 5%, the conversion rate drops to 86%. In addition, studies have also found that the enzyme-containing cells can still maintain high catalytic activity after immobilization. Jagtap S S et al. found that galactitol dehydrogenase derived from Rhizobium pea can also use galactitol as a substrate to generate D-tagatose. Studies have found that this galactitol dehydrogenase has higher specific activity than other reported galactitol dehydrogenases. However, because galactitol is relatively expensive, this method is not suitable for industrial production and has little commercial application value.
2.2 Using D-galactose as raw material to produce D-tagatose
Compared with the higher cost of galactitol oxidation to produce D-tagatose, Cheetham PS et al. first reported the use of L-arabinose isomerase to catalyze the isomerization of D-galactose to produce D-tagatose in 1993. The sugar method has more industrial prospects. Lim B C et al. used Bacillus type L-arabinose isomerase to catalyze the reaction of D-galactose isomerization to D-tagatose. The research results show that the highest yield is 11.5g/Lh under the condition of pH 8.5~9.060°C and 0.4mmol/L boric acid, which is 1.5 times higher than that without boric acid.
You may like
Related articles And Qustion
See also
Lastest Price from D-tagatose manufacturers

US $0.00/G2025-04-21
- CAS:
- 87-81-0
- Min. Order:
- 10G
- Purity:
- 98%min
- Supply Ability:
- 30kg/month

US $1.00/kg2025-04-21
- CAS:
- 87-81-0
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 10 mt