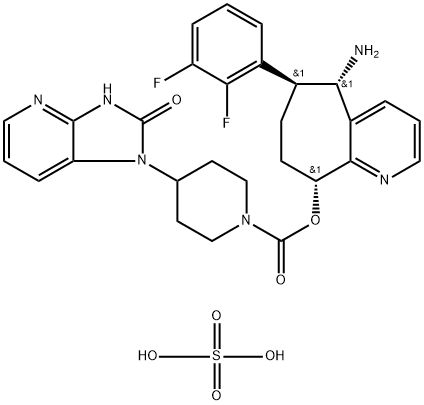
Synthesis of Rimegepant Sulfate
Synthesis of Rimegepant Sulfate
Rimegepant Sulfate is prepared by combining the intermediate compounds Azide and p-nitrocarbamate to form a carbamate intermediate, which is finally crystallised by the addition of warm sulfuric acid. The specific synthesis steps are as follows:
Step 1: preparation of Rimegepant Intermediate Azide
Began with asymmetric hydrogenation of dione 83, which is commercial or can be accessed on large scale by means of a Dieckmann cyclization− decarboxylation from dimethyl 2,3-pyridinedicarboxylate. Enantioselective reduction of dione 83 was accomplished using a chiral rhodium complex identified from a screen of chiral metal catalysts. The exquisite chemoselectivity in this reaction can be attributed to this heterocycle's C9-ketone being more electrophilic than its C5-counterpart. The resulting silyl ether 85 was established using triisopropyl silyl triflate in 74% yield across two steps. Palladium-catalyzed α-arylation was then accomplished with arene 86 resulting in 63% yield and a 12:1 diastereomeric ratio (dr) 87a/87b in favor of the desired transisomer 87a.
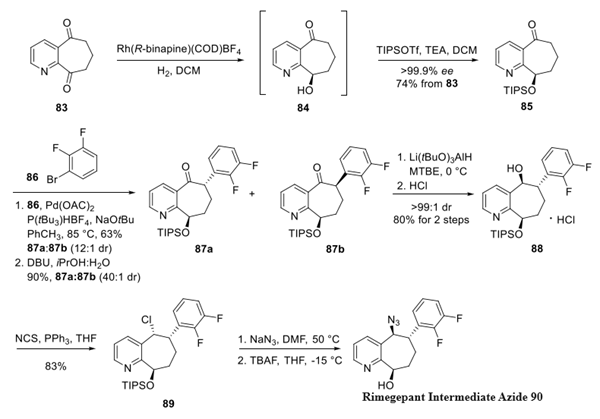
The diasteroselectivity was further improved by base-mediated epimerization resulting in a 90% yield and 40:1 dr. The improvement in this ratio is likely the result of two attributes: the highly crystalline nature of the trans-configuration and presumably the thermodynamically favored isomer relieved strain imposed by the two ring substituents residing on the syn face of the system. After considerable study, ketone 87a was efficiently reduced using lithium tri(t-butoxy aluminum) hydride to arrive at the desired alcohol in a 45:1 dr, which was isolated as the corresponding HCl salt 88. An azide was then installed through the corresponding chloride 89, and removal of the silyl group within this molecule gave the advanced intermediate 90.
Step 2: preparation of Rimegepant Intermediate p-nitrocarbamate
A process route for the synthesis of the remaining region of rimegepant was initiated by a reductive amination of aminopyridine 91 and ketone 92 to deliver piperidine 93. A Buchwald−Hartwig amination reaction employing benzophenone imine followed by careful exposure to citric acid and gentle warming converted chloropyridine 93 to aniline 94 in 80% yield across two steps. Carbonyl diimidazole (CDI) was then used to form cyclic urea 95, making way for Boc group cleavage and a subsequent reaction with chlorocarbonate 97 to establish p-nitrocarbamate 98 in 54% yield.

Step 3: preparation of Rimegepant Sulfate
The union of the two rimegepant intermediates 90 and 98 to form penultimate intermediate 99. Nucleophilic substitution of the p-nitrophenoxy carbamate with alcohol 90 required sodium hexamethyldisilazane (NaHMDS) in chilled DMF to produce carbamate 99 in a satisfactory 73% yield. Azide reduction completed the synthesis of rimegepant. An extensive salt screening was performed on the API in which a hemi-sulfate salt was later identified as the proper commercial form. To prepare this form, rimegepant was dissolved in ethanol and water (3:1) before the slow addition of warm H2SO4. These conditions delivered rimegepant sulfate as a crystalline hemi-sulfate salt in the stoichiometry specified.

You may like
See also

US $0.00-0.00/kg2025-08-05
- CAS:
- 1374024-48-2
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 500kg

US $0.00/kg2025-06-02
- CAS:
- 1374024-48-2
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 1000kg