Synthesis of Nucleic Acid
What is Nucleic Acid?
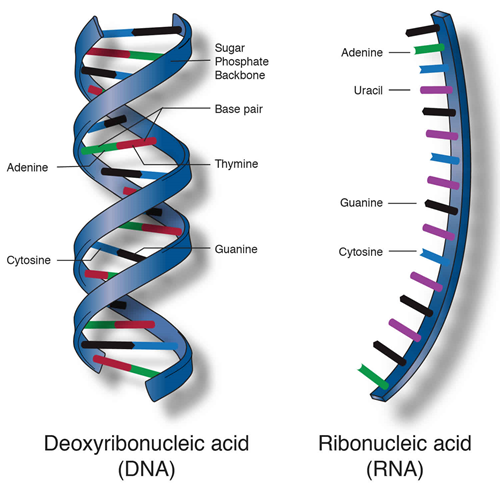
Nucleic acids are biopolymers or biomolecules essential to all known forms of life. It primary function is to store and express genomic information. It consists of two forms, Deoxyribonucleic Acid (DNA) and Ribonucleic Acid (RNA). DNA encodes the information that cells need to make proteins, while RNA, in different molecular forms, performs a variety of roles in the cell, including the synthesis of proteins.
Synthesis
The first step in Nucleic Acid synthesis is the formation of purine and pyrimidine ribonucleotides. There are two endogenous pathways for Nucleic Acid Synthesis: one that regenerates Nucleic Acid from small molecules such as Carbon Dioxide, Amino Acids, and Ribose, which has a high energy cost, and a ‘salvage’ pathway that has a lower energy cost. Purine bases and pyrimidine nucleosides produced by the breakdown of nucleic acids and nucleotide cofactors are rescued within the cell, and the resulting nucleotides can be incorporated into nucleic acids. In most cells, the salvage process is more important, and the ribonucleotides recovered in this way exert feedback control on the de novo pathway.
Currently, Nucleic Acid synthesis is typically performed using automated DNA/RNA synthesizers that perform phosphoramidite chemistry on a solid support (controlled pore glass). Phosphoramidites extracted from protected nucleosides are coupled to the nucleic acid chain through a four-step reaction cycle. Upon completion of nucleic acid chain assembly, the nucleic acid chain is cleaved and deprotected from the solid support to yield the final product.
Using the solid phase phosphoramidite method as an example, the main synthesis steps are as follows:
Nucleic acid synthesis is generally directed from the 3′ end to the 5′ end.
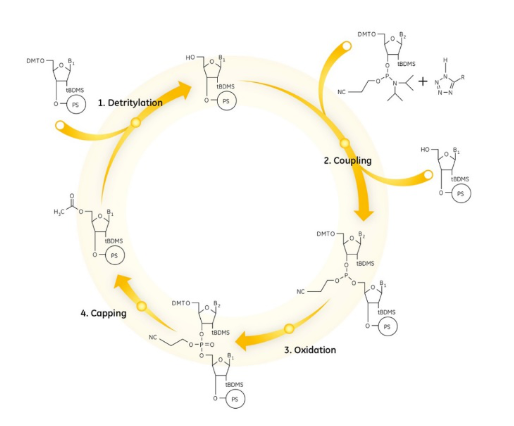
(1) Deprotection
The protecting group DMT on the 5′ hydroxyl group of the nucleotide attached to the solid-phase carrier is removed with trichloroacetic acid or dichloroacetic acid, exposing it for the next step of the reaction.
(2) Coupling
The phosphoramidite monomer is activated with tetrazole to form a highly reactive phosphoramidite tetrazole, which enters the synthesis column and is coupled to the 5′ hydroxyl group of the oligonucleotide attached to the solid-phase carrier in a step with an efficiency of typically more than 98%.
(3) Oxidation
Nucleotide monomer is connected to the oligonucleotide on the solid phase carrier through the phosphite bond during the condensation reaction. However, the phosphite bond is unstable, easy to be hydrolysed by acids and bases, this time the tetrahydrofuran solution of iodine is often used to convert the phosphite into triphosphate, to obtain a stable oligonucleotide.
(4) Encapsulation
In order to prevent a small amount of unreacted (2%) 5′ hydroxyl groups attached to the solid-phase carrier from entering the next cycle, they were closed by acetylation with acetic anhydride, which greatly improved the purity of the final product.
After the above four steps, nucleotides were added one by one to the synthesised oligonucleotide chain. Finally, the oligonucleotides were cut from the solid phase carrier with concentrated ammonia to remove the protective groups from the bases and phosphate groups, followed by purification and quantification.