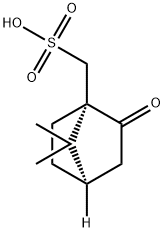
Synthesis of m-toluidine-aniline chirality copolymers induced by D-camphorsulfonic acid
Chiral structures exist widely in biological macromolecules and play a significant role in the living system, such as right-handed protein and duplex DNA. Chiral polymers are structurally characteristic due to the unsymmetrical factors or groups consisting of chiral atoms, which would thus form helical conformations. Chiral polyaniline has been thoroughly investigated by Majidi et al. since 1994. Recently, Yong Yan et al. have successfully prepared right- and left-handed nanopolyanilines utilizing D-camphorsulfonic acid (D-CSA) and L-camphorsulfonic acid (L-CSA) as the dopants. Camphorsulfonic acid, also known as Reychler's acid [1].
Here, we described the synthesis of mtoluidine-aniline copolymers induced by D-CSA utilizing chemical oxidation, and described the expected results, so as to provide a novel approach for transforming the properties and structures of the chiral polyaniline and controlling the chiral conformation by An derivatives and An copolymers.
Synthetic methods
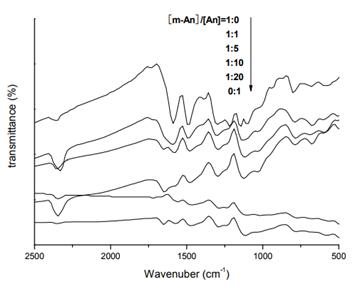
The monomers m-An and An with certain ratio were added into the aqueous solution of D-CSA. The concentration of D-CSA was 2.8mol/L, and the total concentration of the monomers was 0.14 mol/L in the system. Ammonium persulfate aqueous solution was then added when the monomer fully dissolved in D-CSA solution in 1.5 and the molar ratio of ammonium persulfate and monomers was 1:1. The reaction was stirred in cold water bath at 0-5℃, and was then left overnight. The product was separated by centrifugation and dried under vacuum at 60℃ for 48h. The ratio of the mixed monomers was adjusted to 1:0, 1:1, 1:5, 1:10, 1:20 and 0:1, respectively, yielding a series of products.
Instrumentation
FTIR spectra were recorded by Thermo Nicolet Nexus FTIR infrared spectrophotometer, UV-Vis spectra were examined by Shimadzu UV-2550 ultraviolet spectrophotometer, CD spectra were recorded by Chirascan circular dichroism spectrometer from British Applied Optical Physics Co., Ltd., and the micro morphology was observed utilizing FEI Quanta 400 FEG scanning electron microscope.
Results
The copolymerization reaction of m-toluidine and aniline has been induced by D-camphorsulfonic acid. When the total concentrations of the acid, the oxidant and the monomer were maintained, the ratio of two types of monomers was varied to realize the structure and property control of m-toluidine-aniline copolymers. FTIR, UV-Vis, CD and SEM were utilized to characterize the products. The results showed that the synthesized copolymers were of fiber and tube structure with the diameter of approximately 70 nm. The introduction of methyl moiety in the molecular chain of benzene weakened the conjugation and blue shifts were observed in both FTIR and UV-Vis spectra. Besides, CD spectra verified the chirality of the copolymers. Negative cotton effect could be discerned at 400 nm and the chirality inversed when [m-An]/[An]=1:5[3].
Reference
[1]https://encyclopedia2.thefreedictionary.com/d-camphorsulfonic+acid
[2]https://pubchem.ncbi.nlm.nih.gov/compound/d-Camphorsulfonic-acid
[3] Yan, Hai Yan , and K. C. Kou . "Synthesis of M-Toluidine-Aniline Chirality Copolymers Induced by D-Camphorsulfonic Acid." Advanced Materials Research 549(2012):161-166.
You may like
See also
Lastest Price from (1S)-(+)-Camphor-10-sulphonic acid manufacturers

US $9.90/KG2025-04-21
- CAS:
- 3144-16-9
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 5tons

US $10.00/kg2025-04-21
- CAS:
- 3144-16-9
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 20 ton