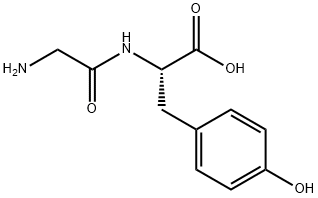
Synthesis of Glycyl-L-tyrosine
L-Tyrosine is an aromatic amino acid and one of the 20 amino acids that synthesize proteins. Tyrosine can be synthesized in the body, so it is a non-essential amino acid. Through a series of catalytic enzymatic hydrolysis in the body, tyrosine can produce catecholamines such as dopamine, norepinephrine, epinephrine, etc., which are nerve mediators of the sympathetic nervous system and central nervous system. Tyrosine can also be catalyzed to form dopamine in melanin cells, and then cyclized and decarboxylated to produce indoloquinone. The polymer of indoloquinone is melanin. Therefore, if the human body lacks tyrosine, it will cause a variety of diseases such as mental illness, neurological disease, psychological disease and albinism.
When the human body lacks L-tyrosine, it must be supplemented in time, but the traditional amino acid nutrient solution does not contain tyrosine, mainly because the solubility of tyrosine in water is very low, and the solubility of tyrosine at 25 ° C is 0.045g / mL. With the investigation of the transformation mechanism of peptides and dipeptides in vivo, it was found that after tyrosine synthesized dipeptide, the solubility and stability in water were greatly increased, and it was easier to absorb in the gastrointestinal tract than monomeric amino acids. Tissues can be rapidly hydrolyzed into free amino acids by proteases to function. Glycyl-L-tyrosine is easily soluble in water and has stable properties during temperature sterilization and long-term storage. Therefore, compared with similar products, Glycyl-L-tyrosine has obvious advantages and broad market prospects.
There are several methods reported in the literature for the synthesis of Glycyl-L-tyrosine:
1.Acyl chloride method
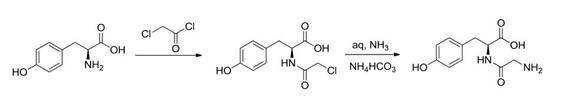
A method was reported in the literature to prepare N-chloroacetyl-L-tyrosine from chloroacetyl chloride and tyrosine or ethyl tyrosine under alkaline conditions, Glycyl-L-tyrosine is then prepared in a solution of ammonia and ammonia bicarbonate The reaction process of chloroacetyl chloride and tyrosine is simple in operation and high in yield, but the final product needs to be separated and purified by column chromatography using a resin column, and then recrystallized in water. The purification is complicated and it is difficult to be industrially applied.
2. Carbodiimidazole method
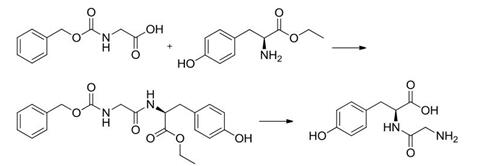
Glycine is protected with benzyloxycarbonyl, then N, N'-carbonyldiimidazole is used as a condensing agent to perform condensation reaction with ethyl tyrosine ethyl ester. Then, the benzyloxycarbonyl group and the ester group are removed by hydrolysis to obtain Glycyl-L-tyrosine.
References
[1] Wu S, Zhang C, Cao R, et al. pH-Dependent reactivity for glycyl-L-tyrosine in carboxypeptidase-A-catalyzed hydrolysis[J]. The Journal of Physical Chemistry B, 2011, 115(34): 10360-10367.
[2] Smirnov V I, Badelin V G. Influence of the composition of water–organic mixtures and of organic solvents properties on solvation of glycyl-L-tyrosine at 298.15 K[J]. Journal of Molecular Liquids, 2014, 195: 139-144.
[3] Bera B K, Ray S, Mondal S, et al. Kinetic and Mechanistic Studies on the Interaction of Glycyl-L-alanine, Glycyl-L-asparagine, and Glycyl-L-tyrosine with Hydroxopentaaquarhodium (III) Ion[J]. Journal of Chemistry, 2013, 2013.
[4] Grayson I, Kessler C. Modern applications of amino acids and dipeptides[J]. Chimica Oggi–Chemistry today, 2015, 33: 46-51.
[5] Lahtinen M, Turhanen P, Saari A, et al. Gly-L-Tyr[J]. Acta Cryst, 2011, 67: C273-C274
You may like
Related articles And Qustion
See also
Lastest Price from N-Glycyl-L-tyrosine manufacturers

US $0.00-0.00/Kg/Drum2025-04-21
- CAS:
- 658-79-7
- Min. Order:
- 25KG
- Purity:
- 99%min
- Supply Ability:
- 3500kg/month

US $6.00/kg2025-04-21
- CAS:
- 658-79-7
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 2000KG/Month