Synthesis of Berotralstat Dihydrochloride
Synthesis of Berotralstat Dihydrochloride
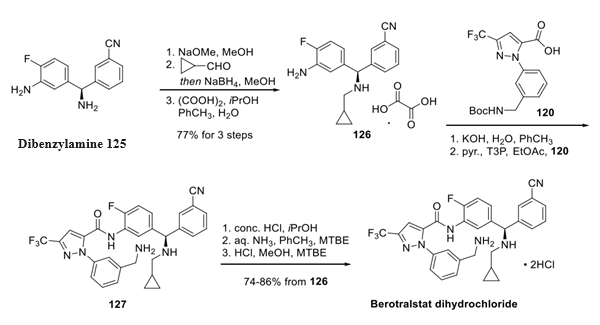
The method for the synthesis of berotralstat dihydrochloride relies on two fragments, berotralstat carboxylic acidFragment and chiral dibenzylamine. The synthesis was carried out in three steps as follows:
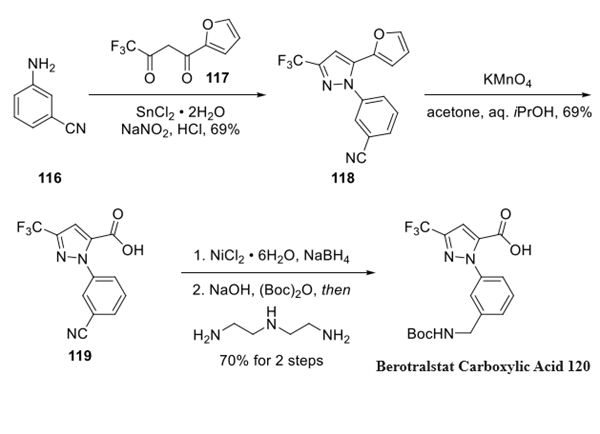
Step 1: Preparation of Berotralstat Carboxylic Acid Fragment
Upon diazotization and oxidation state adjustment mediated by stannous chloride, aniline 116 underwent regioselective cyclative condensation with diketone 117 to afford pyrazole 118.Oxidative cleavage of the furan provided carboxylic acid 119 in good yield. Notwithstanding atom economy considerations , this reaction represents a clever utilization of furan as a latent carboxylic acid surrogate. Reduction of the nitrile was accomplished with nickel boride, which was prepared in situ, and under basic conditions, the protective carbamate was established to arrive at acid 120.
Step 2: Preparation of Berotralstat Dibenzylamine Fragment
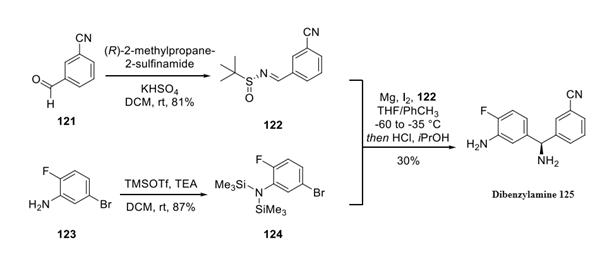
Concurrently, the dibenzylamine fragment 125 was prepared through a condensation of (R)-tertbutylsulfinamide with commercial aldehyde 121 in good yield. 130 This Ellman product underwent nucleophilic attack from the Grignard reagent of aryl bromide 124, which interestingly needed to be protected as the hexamethylsilazane (prepared from arene 123) in order to carry the latent aniline functionality through the sequence. The conversion of bromide 124 to the corresponding Grignard reagent followed by subjection to 122 and subsequent acidic removal of the auxiliary and recrystallization from isopropanol furnished amine 125 in 30% yield and unreported enantiomeric purity.
Step 3: Preparation of Berotralstat Dibenzylamine
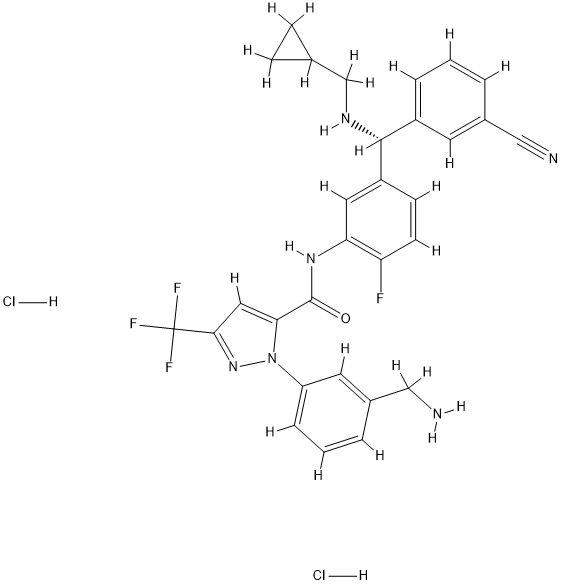
To complete the synthesis, dibenzylamine 125 was treated with base prior to a reductive amination with cyclopropylcarboxaldehyde, and the resulting amine was treated with isopropanolic oxalic acid to furnish cyclopropylamine 126 as the oxalate salt. Salt break upon exposure to base followed by amide coupling conditions with acid 120 established the molecular framework of berotralstat as a free base (127). Lastly, acid−base treatment followed by subjection to methanolic HCl furnished berotralstat dihydrochloride as the bis-HCl salt, which was formed in good yield across five steps from oxalate salt 126.