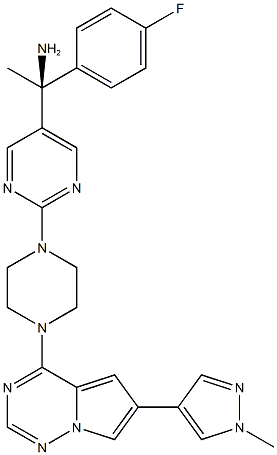
Synthesis of Avapritinib
Synthesis of Avapritinib
The synthesis of Avapritinib (BLU-285) occurs in two steps and requires the involvement of the intermediate Avapritinib Amine Salt. The specific synthesis steps are as follows:
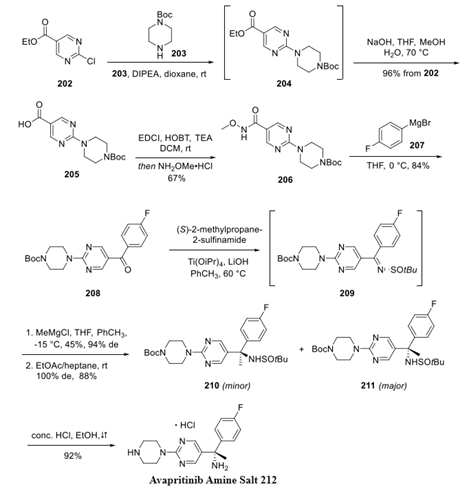
Step 1: Preparation of Avapritinib Amine Salt
Pyrimidyl chloride 202 was substituted with piperazine 203, and the resulting ester 204 was saponified to deliver acid 205. Activation as the Weinreb amide 206 prior to subjection to Grignard 207 furnished ketone 208, which was then activated as the Ellman auxiliary using (S)-2-methylpropane-2-sulfinamide prior to the reaction with chilled methylmagnesium chloride in a mixture of THF and toluene. Although the yield of this reaction was moderate, excellent diastereoselectivity (94% de) was achieved, and a mixture of sulfinamides 210 and 211 was generated. The mixture was further resolved by crystallization in EtOAc/heptane to deliver 211 as the major product, avoiding the need for chromatography. Treatment of aminopyrimidine 211 to acidic conditions in refluxing ethanol removed both the sulfinamide and carbamate functional groups to construct the enantiopure amine 212 as the monohydrochloride salt in excellent yield.
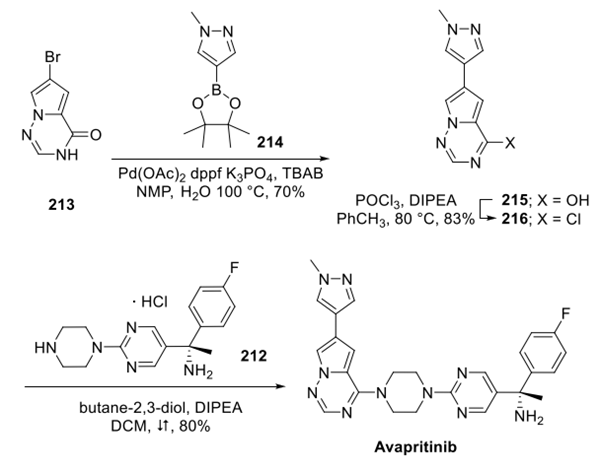
Step 2: Preparation of Avapritinib
Concurrently, bromide 213 was coupled with pyrazole boronate 214, giving rise to triazinone 215, which was converted to the corresponding chloride using phosphorus oxychloride and base in warm toluene. This aryl chloride underwent smooth introduction of piperazine 212 under basic conditions in refluxing dichloromethane to deliver avapritinib in excellent yield.
You may like
See also
Lastest Price from Avapritinib manufacturers

US $0.00/g2025-01-13
- CAS:
- 1703793-34-3
- Min. Order:
- 1g
- Purity:
- More Than 99%
- Supply Ability:
- 50kg/Month

US $0.00/g2025-01-13
- CAS:
- 1703793-34-3
- Min. Order:
- 1g
- Purity:
- More Than 99%
- Supply Ability:
- 50kg/Month