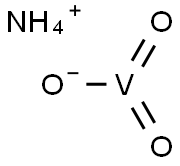
Synthesis of Ammonium metavanadate
If a smaller volume of liquid is desired, V3O5 is dissolved in NasCO3 as NaVO3, and NH4VO3 is precipitated from this solution by addition of NH4C1. Thus 25 g. of V3O5 is added, in small portions and with stirring, to a boiling solution of 17.5 g. of anhydrous Na3CO3 in 125 ml. of H3O. After the CO2 evolution has subsided, saturated KMnO4 solution is added to the reaction mixture in an amount just sufficient to discharge the green-blue color stemming from partial reduction of the vanadium. Undissolved V3O5 and MnO3 are carefully suctionfiltered on a fine-pore fritted glass filter until the filtrate is completely clear. The residue is washed with H2O until H3O3 no longer gives a positive reaction for vanadium. The filtrate, which amounts to about 125-150 ml., is heated to 60°C and then poured all at once into a hot solution of 75 g. of NH4C1 in 125 ml. of H2O.
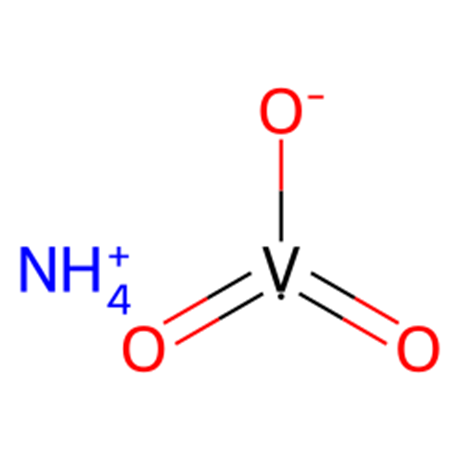
Precipitation of NH4VO3 starts immediately and is complete after a few hours. Some of the salt adheres fairly tenaciously to the glass walls. The salt is suction-filtered and washed with small portions of H3O until the washings are free of chloride ion. It is then dried in air at a temperature below 40 °C. An almost purewhite salt, still containing some Na (about 0.3% NaCl) is obtained in 80% yield.
The NH4VO3 can be recrystallized under similar conditions.
For example, 25 g. of the salt is dissolved in a solution of 16 g. of
NasCO3 in 125 ml. of H3O with mild heating (to 30-40 °C). The
mixture is then carefully filtered and precipitated at once with
NH4C1 as described above.
Lastest Price from Ammonium metavanadate manufacturers

US $10.00/KG2025-04-21
- CAS:
- 7803-55-6
- Min. Order:
- 100KG
- Purity:
- 99%
- Supply Ability:
- 100 mt

US $0.00-0.00/kg2025-03-07
- CAS:
- 7803-55-6
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 100tons