Synthesis of 2,3-Dihydrothiophene
2,3-Dihydrothiophene, also known as 2-thiolene, is a five-membered, nonaromatic, partially saturated sulfur heterocycle comprised of four carbon atoms and one sulfur atom, derived by the partial reduction of one of the two double bonds of thiophene. The two carbon atoms of 2,3-dihydrothiophene are sp3 hybridized and the other two are sp2 hybridized. The bond angle C-S-C is 95 degrees, which is a little larger than the angle in thiophene, 92.17 degrees.
Physical Properties
2,3-Dihydrothiophene is a colorless liquid with a bp of 48°C/100 mm pressure, an mp of 110.2°C, and is soluble in most of the organic solvents. Heat of formation is 12.76 kcal/mol. UV (MeOH), λnm: 236, 262. 1H NMR (CS2), δ (ppm): C2–H, 6.06; C3–H, 5.48; C4–H, 3.08; C5–H, 2.62.
Synthesis
The parent 2,3-dihydrothiophene has been prepared by flash vacuum pyrolysis (FVP) of 2-acetoxytetrahydrothiophene at 400°C under 10–4 torr pressure for 2 h in 86% yield.
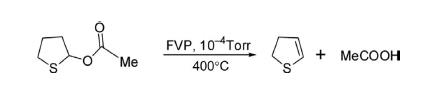
Attempts were made to reduce the drastic reaction conditions to obtain the desired compound. The synthesis of 2,3-dihydrothiophene was initially reported by G. Sosnovsky in 1961by pyrolysis of benzoyloxytetrahydrothiophene at 100°C in 80% yield. It was also obtained by just boiling the 2-benoyloxytetrahydrothiophene in tert-butanol for 100 h in 64% yield. The yield of the product was improved 82% by heating 2-benoyloxytetrahydrothiophene at 110–140°C under 10 mm pressure.
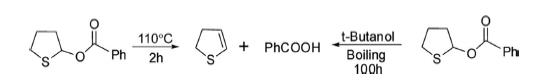
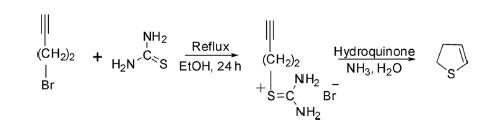
2,3-Dihydrothiophene has also been prepared by the reaction of 4-bromo-1-butyne with thiourea in boiling ethanol, which gave S-1-butynethiourea salt as an intermediate, which on treatment with aqueous ammonia and hydroquinone transformed to 2,3-dihydrothiophene.