Properties and Synthesis of 1,2,3-Thiadiazole
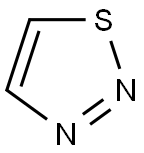
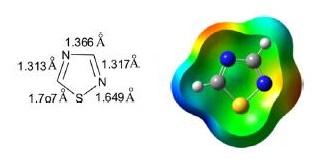
1,2,3-Thiadiazole is a five-membered, π-excessive, stable, almost planar, unsaturated, conjugated, heteroaromatic comprised of one sulfur atom, two adjacent nitrogen atoms (S–N–N), and two carbon atoms. There are four possible noninterconvertible regioisomeric structures depending on the relative position of heteroatoms.
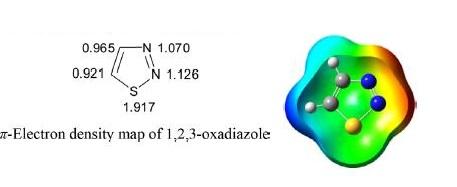
The π electron density map revealed that electron density at sulfur is maximum followed by nitrogen. The carbon atoms C4 and C5 are electron deficient and as a result electrophilic substitution at these positions is not facile, while the preferential site for nucleophilic substitution is C5 because of low electron density at this position. Quaternization with dimethyl sulfate gave a mixture of 2- and 3-methyl-1,2,3-thiadiazole.
Uses
1,2,3-Thiadiazoles have broader applications as insecticide synergists, cross-linked polymer compounds, and herbicides. Besides agro-applications there are other applications such as sedative, antibacterial and antibiotic, antiviral, and neurodegenerative.
Synthesis
There are various synthetic approaches for the construction of 1,2,3-thiadiazoles and the major protocols are described next.
Hurd-Mori Method:
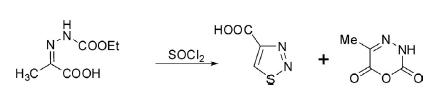
The widely acceptable and effective route for the synthesis of 1,2,3-thiadiazoles is documented through intramolecular cyclization of hydrazones in the presence of SOCl2 or S2Cl2. The reaction of 2[(ethoxycarbonyl)hydrazono] propionic acid with thionyl chloride produced 1,2,3-thiadiazolo-4-carboxylic acid in 53% yield with a trace of 5-methyl-2H-1,3,4-oxadiazine-2,6(3H)-dione as a side product.
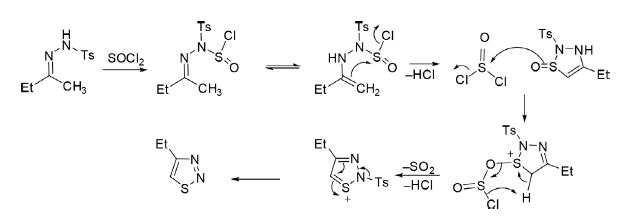
Hydrazone derived from the reaction of tosyl hydrazine with ethyl methyl ketone on treatment with thionyl chloride underwent cyclization to 4,5-disubstituted 1,2,3-thiadiazole. The plausible mechanism of the reaction is depicted in the following scheme.
Physical Properties
The parent 1,2,3-thiadiazole is a yellow-colored liquid with a bp of 157°C. It is a weak base, thermally stable, and soluble in water and most of the organic solvents such as alcohol, ether, DCM, and chloroform.
Chemical Reactivity
As is evident from the electron density map on the ring atoms, both C4 and C5 carbon atoms have the least electron density compared to nitrogen, making them resistant to electrophilic attack on carbon atoms but facile to nitrogen atoms. However, nucleophilic substitution is facile to carbon atoms.