Synthesis and Characteristics of Polycarbomethylsilane
Introduction

Figure 1. Properties of Polycarbomethylsilane
Polycarbosilanes (PCSs) with the general formula -[(R1) (R2) SieC(R3) (R4)]n- (Ri: i (1-4)=H or organic groups) with alternating silicon-to-carbon sequences in their backbones are still the essential polymeric precursors to manufacture silicon carbide (SiC) ceramics via a polymer-derived ceramic (PDC) method. Several routes for manufacturing one-dimensional SiC structures are available so far, e.g., chemical vapor deposition, carbothermic reduction, arc discharge, laser ablation, and combustion synthesis. For example, the combustion synthesis route can prepare SiC nanowires through the highly exothermic redox reaction between Si powder and polytetrafluoroethylene, featured with one-pot, a short duration synthesis, and low energy demand. However, the PDC method has attracted considerable attention because of its main advantage of low processing temperatures, enabling the production of ceramics with desirable morphologies, such as particles, fibers, thin films, and ceramic matrix composites (CMCs). For instance, SiC fibers can be fabricated via successive processes, i.e., spinning, curing, and pyrolysis, by using a PCSs.1-5
To date, there have been several methods for the synthesis of PCSs. For example, the thermal rearrangement and radical decomposition of polysilanes (i.e., the Kumada reaction), Grignard polycondensation reaction of chlorosilanes, catalyzed ring-opening polymerization of 1,3- disilacyclobutanes by Pt-containing complexes, dehydrocoupling reaction of trimethylsilane or hydrosilylation of vinylhydridosilanes. However, the development of safe, efficient, and economical processes for producing PCSs is still an attractive research topic.6
Among PCSs, polycarbomethylsilane is the most commonly used PCS precursor for manufacturing SiC ceramics and has been widely utilized since a pioneering study by Yajima et al. Currently, commercial polycarbomethylsilane is usually fabricated by the Kumada reaction of polydimethylsilane (PDMS) in an autoclave at high temperatures (≥ 400 oC) for a long time (≥ 15 h). Some flammable gaseous byproducts (including CH4, (CH3)3SiH, and SiH4) could result in a high pressure (>10 MPa) inside the autoclave, leading to an unsafe and inconvenient operation. In addition, PDMS must be synthesized in advance through a Wurtz dichlorination coupling reaction using dimethyldichlorosilane (DMDCS, hereafter referred to as SiMe2Cl2) and sodium metal. This two-step process for the synthesis of polycarbomethylsilane may also give rise to implicit problems, such as a low yield (<50%), large energy consumption, and low production rate. Addressing the unfavorable situation in the thermal decomposition/rearrangement procedure, several studies have added a catalyst to PDMS, for example, polyborodimethylsiloxane or a heterogeneous solid acid (metallic chlorides or zeolites) to convert PDMS into polycarbomethylsilane under gentle reaction conditions. However, obtaining the polycarbomethylsilane product with a suitable molecular weight and avoiding possible contamination from the catalyst are crucial issues. Furthermore, the overall production process of polycarbomethylsilane still consists of two steps since PDMS must be prepared beforehand.7-9
Synthesis of Polycarbomethylsilane
The SiMe2Cl2-to-TiCp2Cl2 molar ratio (referred to as the R value hereafter) in the synthesis ranged from 10 to 60. The amount of sodium metal used was 1.5 times higher than the amount of the stoichiometric dechlorination reaction of SiMe2Cl2 and TiCp2Cl2. In all the experiments, the amount of TiCp2Cl2 was fixed at 5.00 x 10-3 mol. Taking R=40 as an example, 250 mL of toluene, 1.24 g of TiCp2Cl2, and 4.72 g of sodium metal (0.205 mol) were added to the flask. The mixture was heated to 100 oC in a nitrogen atmosphere under reflux. Under continued heating and stirring, the sodium metal melted into droplets dispersed in toluene, and then 25.8 g of SiMe2Cl2 (0.200 mol) was added dropwise to the closed systemat a feeding rate of ~1 mL/min to participate in the synthesis. During this time period, the suspension was maintained at~100 oC and stirred continuously at a revolving speed of ~300 rpm for proper homogenization, and its color changed to bright red. The acidity of the suspension decreased as the reaction proceeded. The reaction was completed when the acidity remained constant (~6-8 h), which was determined by an acid-base indicator. Then, hydrogen was introduced into the reaction system to ensure the termination of the reaction and to stabilize the product. The residual sodium metal was eliminated with methanol. The precipitate, likely containing NaCl, PDMS, and NaOCH3, was removed from the suspension by suction filtration. The toluene was separated from the filtrated suspension by a rotary evaporator, and a solid with a light brown color was obtained. After rinsing twice with nhexane and deionized water successively and then drying under vacuum, the as-synthesized product was obtained. The as-synthesized product was free of chlorine as determined by the precipitation titration method.10
Reaction Mechanism
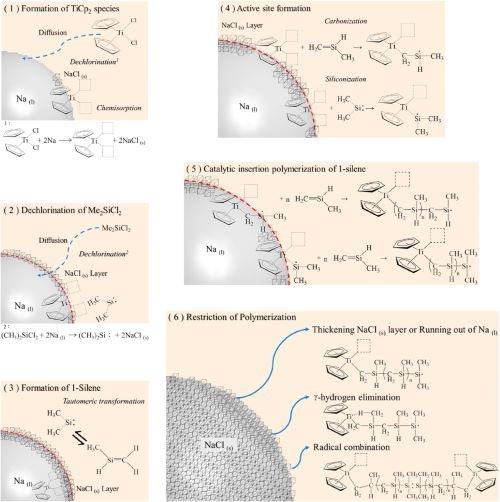
Figure 2. Proposed reaction mechanism.
By combining the experimental procedures and the analysis results of this study and referring to the coordination insertion polymerization of 1-olefin by metallocene, a possible mechanism for the synthesis of polycarbomethylsilane was proposed, which is illustrated in Fig. 2 and divided into six steps as follows.11-12
(1)TiCp2Cl2 is dechlorinated by reacting with a Na(l) droplet, which leads to the formation of Cp2Ti species and NaCl(s). Then, the titanocene species chemisorbs onthe surface of the Na(l) droplets resulting from the interaction between the free electrons in sodium metal and the unlocalized electrons in cyclopentadienyl groups.
(2)With the continuous addition of SiMe2Cl2 into the reactor, SiMe2Cl2 diffuses and penetrates the toluene liquid film and the NaCl(s) layer. Upon reaching the surface of the Na(l) droplet, the dechlorination reaction of SiMe2Cl2 takes place to form dimethylsilylene (i.e., Me2Si) and NaCl(s). Because both the elemental analysis of the as-synthesized product and EDS analysis of the as-prepared fiber did not detect any chlorine, it is assumed that the chlorine in SiMe2Cl2 is removed entirely in the dechlorination reaction with Na(l).
(3)Immediately, the tautomeric conversion from Me2Si: into methylsilene (MeHSi=CH2) occurs in the presence of titanocene species due to thermodynamic and kinetic preferences.
(4)Afterward, the coordination insertion polymerization is initiated by the formation of an active site via titanium siliconization or carbonization, thus exerting a catalytic effect of the titanocene species. MeHSi=CH2 and Me2Si diffuse from the NaCl(s) layer to the active site of the carbonized/siliconized TiCp2 to participate in the insert polymerization reaction. The successive steps of electron appealing and transfer progress rapidly, including the absorption of a 1-silene monomer of MeHSi=CH2 on the active site, the formation of the coordination complex, the insertion of the monomer into the TieC bond, and the regeneration of the active site, as analogously illustrated elsewhere.
(5)The regeneration of the active site, in which MeHSi=CH2 is trapped, results in a series of one-by-one insertions of MeHSi=CH2 into PMCS accompanied by linear-chain propagation and elongation.
(6)The adverse factors of the insertion polymerization result from the diffusion barrier of the thickening NaCl(s) layer, depletion of Na(l) droplets, occupation of the active sites (such as g-hydrogen elimination), and radical combination, which may cause the breakdown of chemisorption of the polymeric chain and even give rise to the termination of the reaction.
The above heterogeneous mechanism is controlled by both diffusion-limited and reaction-limited factors. The competition between the two factors may explain the effects of the R value on the product yield and the polymerization effectiveness. Since the dechlorination reaction gives rise to the consumption of sodium metal and the accumulation of NaCl(s) on the surface of the Na(l) droplets, diffusion of SiMe2Cl2 to the Na(l) surface may be prevented by the decreasing droplet size and free surface of the Na(l). On the other hand, the preferential polymerization of MeHSi=CH2 could be restricted by the series of steps and the turnover number of active sites. In addition to the catalytic insertion polymerization reaction between titanocene and MeHSi=CH2, another parallel reaction, i.e., the direct polymerization of Me2Si to produce the PDMS byproduct, can also occur. This is because a certain amount of Me2Si is present in the reaction system, even though the tautomer MeHSi=CH2 possesses kinetic and thermodynamic advantages.
References
1.Mera G, Gallei M, Bernard S, Ionescu E. Ceramic nanocomposites from tailor-made preceramic polymers. Nonomaterials 2015;5(2):468-540.
2.Ding D. Processing, properties and applications of ceramic matrix composites, SiCf/SiC; an overview. In: Low IM, editor. Advances in ceramic matrix composites. 2nd. ed. Cambridge: Woodhead;2018.p.9-25.
3.Ichikawa H. Polymer-derived ceramic fibers. Annu Rev Mater Res 2016;46:335-56.
4.Mera M, Ionescu E. Silicon-containing preceramic polymers. In: Encyclopedia of polymer science and technology. Hoboken: Wiley;2013.0471440264.pst591.
5.Lee YJ, Lee JH, Kim SR, Kwon WT, Oh H, Klepeis J-hP, et al. Synthesis and characterization of novel preceramic polymer. J Mater Sci 2010;45:1025-31.
6.Weinmann M, Ionescu E, Riedel R, Aldinger F. Precursorderived ceramics. In: Somiya S, editor. Handbook of advanced ceramics: materials, applications, processing, and properties. 2nd. Amsterdam: Elsevier;2013.p.1025-101.
7.Yajima S, Hasegawa Y, Okamura K, Matsuzawa T. Development of high tensile strength silicon carbide fibre using an organosilicon polymer precursor. Nature 1978;273:52507.
8.Cheng X, Xie Z, Song Y, Xiao J, Wang Y. Structure and properties of polycarbosilane synthesized from polydimethylsilane under high pressure. J Appl Polym Sci 2006;99:1188-94.
9.Kim Y, Shin DG, Kim HR, Park HS, Riu DH. Preparation of polycarbosilane using a catalytic process and its practical uses. Key Eng Mater 2005;287:108-11.
10.Peng, C. H., & Hwang, C. C. (2020). Synthesis and characteristics of polycarbomethylsilane via a one-pot approach. Journal of Materials Research and Technology, 9(6), 15838-15848.
11.Shimiri A, Chakrabatri MH, Jahan S, Hussain MA, Kaminsky W, Aravind PV, et al. The influence of ZieglerNatta and metallocene catalysts on polyolefin structure, properties, and processing ability. Mater 2014;7(7):5069-108.
12.Kaminsky W. Olefin polymerization catalyzed by metallocene. Adv Catal 2001;46:89-159.
Related articles And Qustion
Lastest Price from POLYCARBOMETHYLSILANE manufacturers

US $120.00/kg2025-04-15
- CAS:
- 62306-27-8
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 20ton

US $5.60-3.20/kg2025-02-13
- CAS:
- 62306-27-8
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 10 tons