Synthesis and Bioactivity of 2'-Deoxyadenosine
Generally speaking
2'-Deoxyadenosine is a natural deoxyribonucleoside, which is a structural fragment of deoxyribonucleic acid DNA. Like other natural nucleosides, it is involved in the transmission of genetic information in almost all biological cells, affecting protein synthesis and polysaccharide. It plays a very important role in regulating the growth, proliferation, differentiation and inhibition of cells in vivo. It is not only an important raw material for genetic medicine and genetic engineering research, but also has good physiological activity. 2'-deoxyadenosine can inhibit the release of insulin induced by sugar, and can reduce specific phosphodiesterase inhibitors. Or adenosyl cyclase activator to promote insulin secretion.

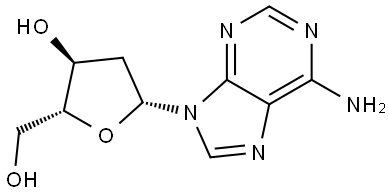
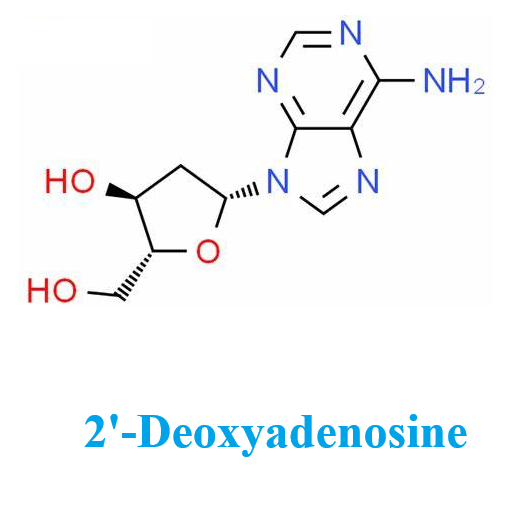
Fig. 1 The structure of 2'-Deoxyadenosine.
Synthetic routes
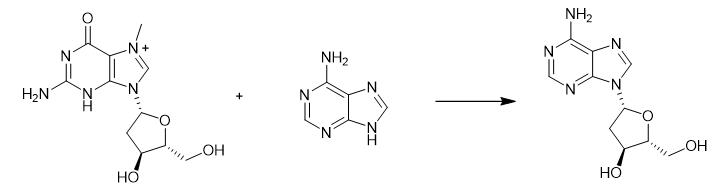
Fig. 2 The synthetic method 1 of 2'-Deoxyadenosine.
Dissolve adenine (236 mg, 1.75 mmol) in 100 mL of 50 mM Tris-HCl buffer (pH 7.5) under heating and cool the solution to room temperature. Add potassium dihydrophosphate (119 mg, 0.875 mmol) and the reactant (1.074 g, 2.62 mmol) to the solution. Dilute the resulting mixture with 50 mM Tris-HCl buffer (pH 7.5) to a volume of 350 mL. Add solutions containing enzymes E. coli PNP [10 μL of 32mg/mL solution of E. coli PNP (2.95 U) ] and E. coli TP [10 μL of 17.2 mg/mL solution of E. coli TP diluted 10-fold (1.2 U) ] to the mixture using a dispenser at room temperature. Stir the resulting mixture for 5 min and allow to stand overnight at room temperature without stirring. Filter the reaction mixture through a Phenomenex membrane (0.2 m, 47mm) and concentrate under vacuum to a volume of ca. 50 mL. Filter the suspension from the precipitate of 7-methylguanine through a Phenomenex membrane (0.2 m, 47mm) and concentrate under vacuum to near dryness. Dissolve the residue in ethanol (50 mL) and add silica gel (20 mL) to the solution. Evaporate the resulting mixture to dryness, co-evaporate with ethanol (2 X 50 mL) and apply the dry residue on a chromatographic column (4 X 10 cm) packed with silica gel (150 mL) for purification. Wash the column with a mixture of dichloromethane and ethanol (95 :5 v/v, 200 mL). Elute the product with dichloromethane :ethanol (90 :10 v/v) and dichloromethane :ethanol (85 :15 v/v). Collect the fractions containing the product and evaporate under vacuum to dryness. Purify the resulting residue on a chromatographic column (2 X 10 cm) packed with silica gel (50 mL). Wash the column with adichloromethane:ethanol mixture (95 :5 v/v, 200 mL). Elute the product with dichloromethane :ethanol (90 :10 v/v). Collect the fractions containing the product. Evaporate the product under vacuum, co-evaporate 5 times with dichloromethane and dry on a vacuum pump at room temperature for 1 hour. [1].
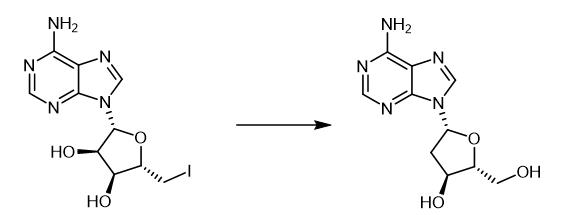
Fig. 3 The synthetic method 2 of 2'-Deoxyadenosine.
In a 5 mL screwcap vial (Wheaton reactor?), provided with a stir bar, a heterogeneous solution in 5 mL water containing the starting water-soluble compound (10 mM), (TMS)3SiH (1.2 equiv), and 2-mercaptoethanol (0.3 equiv) was deoxygenated with an Ar stream for ten minutes. After that, ACCN (1,1-azobis(cyclohexanecarbonitrile), 0.015mmol, 3.6 mg) was added, the vial closed tightly and placed in a thermostatically-controlled metal rack (100.MALE. C) for 4 hours. After the reaction time elapsed, the solution was allowed to reach ambient temperature. Pentane or hexane was added to the crude aqueous reaction mixture and extracted (twice) to separate silane and initiator by-products. The water phase was analyzed by HPLC, using a previously constructed calibration curve for the product to give the reaction yield [2].
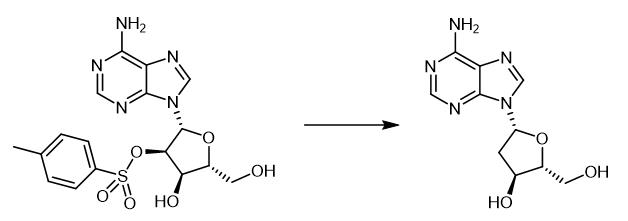
Fig. 4 The synthetic method 3 of 2'-Deoxyadenosine.
LTBH (1 M in THF; 15 mL, 15 mmol) [Note20]was added by syringe into a stirred solution of 2a (421 mg, 1.00mmol) in dried DMSO (15 mL) under N2 at ambient temperature,and stirring was continued for 18 h. H2O (5 mL) was added cautiously, and the solution was concentrated. The residue was chromatographed [(Dowex 1 x 2 (OH-), H2O] and recrystallized(MeOH) to give 9-(2-deoxy-β-D-threo-pentofuranosyl)adenine3, (247 mg, 98%): 9-(2-deoxy-β-D-threo-pentofuranosyl)adenine (3a), yield 98% mp 220-221°C [Note35] (lit.6a mp 218-220 °C); 1H NMR δ 2.26 (d, J = 14.6 Hz, 1H), 2.75-2.82 (m,1H), 3.58-3.63 (m, 1H), 3.71-3.76 (m, 1H), 3.88-3.92 (m, 1H),4.31-4.36 (m, 1H), 4.70 (t, J = 5.4 Hz, 1H), 5.99 (d, J = 5.4 Hz,1H), 6.26 (d, J = 8.3 Hz, 1H), 7.36 (br s, 2H), 8.15, 8.35 (2 x s,2 x 1H); 13C NMR δ 40.7, 59.9, 69.2, 82.4,85.2, 119.0, 140.0,148.5, 152.2, 156.1; FAB-MS m/z 252 ([M + H+], 10), 157 (100); HRMS (C10H14N5O3) calcd 252.1091, found 252.1090 [3].
Bioactivity
Selectively kills nonneuronal cells
Wakade et al. have demonstrated that adenosine and 2'-deoxyadenosine are toxic to embryonic sympathetic neurons and proposed that purine and pyrimidine metabolism may play a critical role in the growth and development of sympathetic neurons. To extend this hypothesis further, we examined the effects of these nucleosides on two other neuronal populations in the chick embryo, sensory dorsal root ganglion neurons and parasympathetic ciliary ganglion neurons. Now, we show that 2'-deoxyadenosine and adenosine have no visible adverse effect on the viability of either sensory or parasympathetic neurons. Instead, 2'-deoxyadenosine proved to be highly toxic to the nonneuronal cells. The toxic effects of 2'-deoxyadenosine were markedly enhanced by inhibition of adenosine deaminase, In contrast, adenosine was much less toxic to nonneuronal cells than 2'-deoxyadenosine and its effect was not potentiated by inhibition of adenosine deaminase, Priming of pyrimidine pools by exogenous uridine and the specific inhibitor of the nucleoside transporter, nitrobenzylthioinosine, did not protect nonneuronal cells from 2'-deoxyadenosine toxicity. Since phosphorylation of internalized nucleosides was a key step in the initiation of toxicity in sympathetic neurons, adenosine kinase activity was compared in sensory and sympathetic neuronal cultures. The adenosine kinase activity in dorsal root ganglion cultures was only 20% of that in sympathetic ganglion cultures. Furthermore, inhibition of phosphorylation by blocking 2'-deoxyadenosine kinase with iodotubercidin and 5'-amino-5'-deoxyadenosine had no protective effect against 2'-deoxyadenosine toxicity. [H-3]-thymidine incorporation was inhibited over 90% by 2'-deoxyadenosine as early as 6 h following its addition and for up to 4 days, suggesting inhibition of proliferation of nonneuronal cells by 2'-deoxyadenosine, The nucleoside was also able to wipe out already well established nonneuronal cells, leaving behind an enriched population of sensory neurons. The selective vulnerability of nonneuronal cells to 2'-deoxyadenosine offers a convenient and effective tool for removing nonneuronal cells from neuronal cultures as well as providing a new model for studying the mechanisms of nucleoside toxicity [4].
Intracellular metabolism
Related articles And Qustion
Lastest Price from 2'-Deoxyadenosine manufacturers

US $0.00-0.00/kg2025-08-19
- CAS:
- 958-09-8
- Min. Order:
- 0.10000000149011612kg
- Purity:
- 99%HPLC
- Supply Ability:
- 1000T

US $5.00-0.50/KG2025-06-11
- CAS:
- 958-09-8
- Min. Order:
- 0.10000000149011612KG
- Purity:
- 99% hplc
- Supply Ability:
- 5000kg