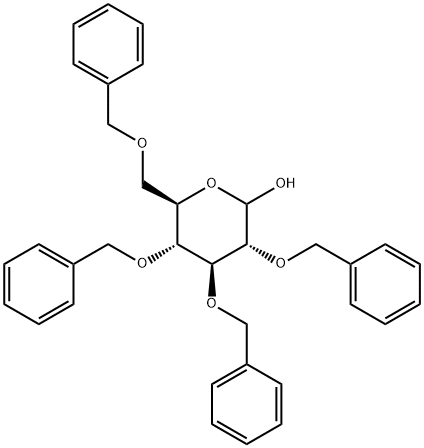
Synthesis and Applications of 2,3,4,6-Tetra-O-benzyl-D-glucopyranose
2,3,4,6-Tetra-O-benzyl-D-glucopyranose is an important D-glucopyranose derivative, which is often used as a pharmaceutical intermediate in the synthesis of derivatives for the treatment of Alzheimer's disease, diabetes, and cancer. is an important D-glucopyranose derivative, which is often used as a pharmaceutical intermediate in the synthesis of derivatives for the treatment of Alzheimer's disease, diabetes, and cancer.

Biosynthetic studies on the alpha-glucosidase inhibitor acarbose
2-epi-5-epi-Valiolone is a cyclization product of the C7 sugar phosphate, sedoheptulose 7-phosphate, involved in the biosynthesis of the aminocyclitol moieties of acarbose, validamycin, and pyralomicin. As part of our investigation into the pathway from 2-epi-5-epi-valiolone to the valienamine moiety of acarbose, we prepared 1-epi-5-epi-(6-2H2)valiolol [(6-2H2)], 5-epi-(6-2H2)valiolol [(6-2H2)], 1-epi-2-epi-5-epi-(6-2H2)valiolol [(6-2H2)] and 2-epi-5-epi-(6-2H2)valiolamine [(6-2H2)]. Compounds (6-2H2) and (6-2H2) were synthesized from 2,3,4,6-tetra-O-benzyl-d-glucopyranose in 10 and seven steps, respectively, whereas (6-2H2) and (6-2H2) were synthesized from 2,3,4,6-tetra-O-benzyl-d-mannopyranose in eight and 10 steps, respectively. 1-epi-5-epi-(6-2H2)Valiolol [(6-2H2)] and 5-epi-(6-2H2)valiolol [(6-2H2)] were prepared from 2,3,4,7-tetra-O-benzyl-5-epi-(6-2H2)valiolone [(6-2H2)]. The latter compound was generated from 2,3,4,6-tetra-O-benzyl-d-glucopyranose in five steps.[1]
Synthesis of 2,3,4,6-Tetra-O-benzyl-D-glucopyranose on the gram scale
Due to their utility in a variety of processes, glycals have received considerable attention in the carbohydrate field. Examples include Danishefsky’s epoxidation/glycosidation methodology, Ferrier-type rearrangements, cyclopropanations, C-glycosylations, and metal-catalyzed transformations. Reports on the use of 2,3,4,6-Tetra-O-benzyl-D-glucopyranose, however, have been limited, perhaps due to its low yielding and problematical synthesis. A new, facile synthesis of 2,3,4,6-Tetra-O-benzyl-D-glucopyranose and its subsequent cyclopropanation has been accomplished by bromination and then base-induced elimination of pent-4-enyl 2,3,4,6-tetra-O-benzyl-d-glucopyranoside. Yields and selectivities are excellent in addition to the ease of formation of the glycal.
All reactions were carried out under argon using oven-dried glassware unless otherwise stated. Purification of products via flash chromatography was conducted using E. Merck Silica Gel 60 using distilled MeOH, CH2Cl2, hexanes, or EtOAc. 1H and 13C NMR spectra were recorded on a Varian INOVA spectrometer at 300 and 75 MHz, respectively, in CDCl3. 4-Penten-1-ol was synthesized using the published procedure.Yields and selectivities are excellent in addition to the ease of formation of the glycal. In summary, Scientists have developed new methodology suitable for the convenient and large-scale synthesis of 2,3,4,6-Tetra-O-benzyl-D-glucopyranose. The cyclopropanation of this glycal has been achieved in excellent yield and selectivity, and the chemistry of this new derivative will be reported in due course.[2]
Preparation and calculated conformations of methyl β-d-galabioside
The glycosyl chlorides of the 3-O-methyland 4-deoxy-4-fluoro O-benzylated derivatives of d-galactopyranose and 2,3,4,6-tetra-O-benzyl-d-glucopyranose were condensed with methyl 2,3,6-tri-O-benzoyl-β-d-galactopyranoside to give, after deprotection, the 3′-O-methyl, 4′-deoxy-4′-fluoro, and 4′-epi derivatives, respectively, of methyl β-d-galabioside. The glycosyl fluorides of 2,3,4-tri-O-benzyl-d-fucopyranose and the 3-deoxy and 4-deoxy O-benzylated derivatives of d-galactopyranose were condensed with methyl 2,3,6-tri-O-benzyl-β-d-galactopyranoside, to give, after deprotection, the 6′-deoxy, 3′-deoxy, and 4′-deoxy derivatives, respectively. The 2′-deoxy derivative was prepared by N-iodosuccinimide-induced condensation of 3,4,6-tri-O-acetyl-d-galactal and followed by deprotection. Treatment of methyl 2,3,6-tri-O-benzoyl-4-O-(2,3-di-O-benzoyl-α-d-galactopyranosyl)-β-d-galactopyranoside with Et2NSF3 (DAST), followed by deprotection, provided the 6′-deoxy-6′-fluoro derivative of derivative 1. Molecular mechanics calculations yielded conformations for derivatives and with small deviations from the calculated conformation for derivative 1 (ΦH/ΨH: −40°/−6°).[3]
Alkylated (e.g., benzylated) glycosyl halides have been used extensively as glycosyl donors in the synthesis of α - glycosides. In the preparation of analogues of methyl β - D - galabioside (1), α - galactosidation of HO - 4 of modified methyl β - D - galactopyranosides using 2,3,4,6-tetra-O-benzyl-d-glucopyranose (2 equiv.) gave higher yields¹ᵇ (70–85%) and was less sensitive to the reaction conditions than when the corresponding galactosyl bromide was used (3 equiv., 40–50% yields). Substitution of an O - benzyl group for O - methyl or fluorine did not alter significantly the properties of the glycosyl chloride (cf. the preparation of 22 and 24), whereas substitution by hydrogen gave less stable chlorides.
References
[1]Arakawa, Kenji et al. “Biosynthetic studies on the alpha-glucosidase inhibitor acarbose: the chemical synthesis of isotopically labeled 2-epi-5-epi-valiolone analogs.” Carbohydrate research vol. 338,20 (2003): 2075-82. doi:10.1016/s0008-6215(03)00315-x
[2]Storkey, Corin M et al. “Synthesis of 2,3,4,6-tetra-O-benzyl-D-glucal on the gram scale. A convenient method for its facile synthesis and subsequent stereoselective cyclopropanation.” Carbohydrate research vol. 339,4 (2004): 897-9. doi:10.1016/j.carres.2003.12.016
[3]Kihlberg, J et al. “Preparation and calculated conformations of the 2'-, 3'-, 4'-, and 6'-deoxy, 3'-O-methyl, 4'-epi, and 4'- and 6'-deoxy-fluoro derivatives of methyl 4-O-alpha-D-galactopyranosyl-beta-D-galactopyranoside (methyl beta-D-galabioside).” Carbohydrate research vol. 185,2 (1989): 171-90. doi:10.1016/0008-6215(89)80033-3
You may like
Lastest Price from 2,3,4,6-Tetra-O-benzyl-D-glucopyranose manufacturers

US $0.00/kg2025-09-01
- CAS:
- 4132-28-9
- Min. Order:
- 1kg
- Purity:
- 99%min
- Supply Ability:
- 20tons
US $0.00/Kg2025-04-21
- CAS:
- 4132-28-9
- Min. Order:
- 1Kg
- Purity:
- 98%
- Supply Ability:
- 20Ton