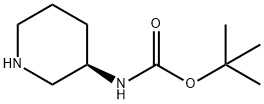
Synthesis and Application of (R)-3-(Boc-Amino)piperidine
General description
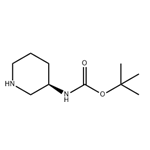
The cas number of (R)-3-(Boc-Amino)piperidine is 309956-78-3; The molecular formula is C10H20N2O2; The molecular weight is 200.28. It is an important intermediate in the synthesis of liraltine and alagliptin benzoate, a type 2 diabetes drug.
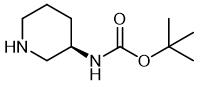
Fig. 1 The structure of (R)-3-(Boc-Amino)piperidine.
Physicochemical property
(R)-3-(Boc-Amino)piperidine is a white powder with a melting point of 121.0 to 125.0 °C. Its boiling point is estimated to be 304.8±31.0 °C and its density is predicted to be 1.02±0.1 g/cm3. It is soluble in ethanol and methanol.
Synthetic routes
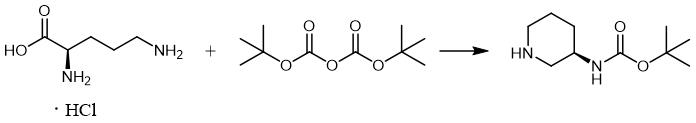
Fig. 2 The synthetic scheme 1 of (R)-3-(Boc-Amino)piperidine.
D-Ornithine hydrochloride (8.43 g, 50 mmol) was slurried in anhydrous methanol (100 mL), saturated with hydrogen chloride and then refluxed for 3 h. The reaction solution was reduced in volume on a rotary evaporator to 1/3 volume and stored in a refrigerator at -20°C overnight. The filtered solid product was washed with minimal amounts of cold methanol and dried in vacuum at 50 °C, obtaining 9.86 g (45 mmol) of crude methyl orthinate dihydrochloride. The crude methyl ester was refluxed with sodium methoxide (6.26 g, 92 mmol) in methanol (120 mL) for 4 h. Ammonium chloride (5.35 g, 0.1 mol) was added and the mixture was allowed to stand overnight. Filtration and concentration gave a thick syrup which was extracted repeatedly with CH2Cl2/MeOH (25 mL, v/v 4/1). The combined filtrates were concentrated, obtaining a white solid, which was dissolved in 25 mL of boiling ethanol (abs), filtered while hot, and stored in the refrigerator overnight. The crystalline product was suction filtered and dried in vacuo [1].
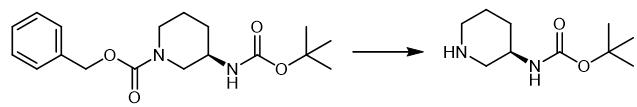
Fig. 3 The synthetic scheme 2 of (R)-3-(Boc-Amino)piperidine.
A mixture of benzyl 3-[(tert-butoxycarbonyl)amino]-1-piperidinecarboxylate (2.25 g, 6.73 mmol) and 10% Pd/C (0.229 g) in ethanol (20 mL) was stirred at room temperature under a hydrogen atmosphere (1 atm) for 14 hrs. The catalyst was removed by filtration through Celite and the filtrate was concentrated under reduced pressure. tert-Butyl 3-piperidinylcarbamate (1.22 g, yield; 91%) was obtained as a solid [2].
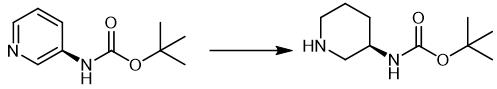
Fig. 4 The synthetic scheme 3 of (R)-3-(Boc-Amino)piperidine.
Manufacturing t-butyl piperidine-3-ylcarbamate Into a solution obtained by dissolving 100 g (0.52 mol) of t-butyl pyridine-3-ylcarbamate in 400 g (6.66 mol) of acetic acid, 30 g of palladium carbon (5%) was added and the resultant was stirred for 12 hours at a hydrogen pressure of 0.6 MPa and at 65°C. After the reaction was finished, palladium carbon was separated by filtration to obtain a reaction solution; palladium carbon was washed with 250 ml of water to obtain a washing liquid; and the aforesaid reaction solution and the washing liquid were mixed. The obtained solution was added dropwise into a solution obtained in advance by dissolving 266 g (6.65 mol) of sodium hydroxide in 500 ml of water. The inner temperature of the mixture during the dropwise addition was set to be 10 to 20°C. After 200 mL of water was further added thereto, the deposited crystals were filtered and washed with 400 ml of water. The obtained crystals were dried to obtain 76.2 g of t-butyl piperidine-3-ylcarbamate as a white crystal, with a yield of 73.8%.
Uses
(R)-3-(Boc-Amino)piperidine is Boc protected (R) -3-ampiperidine, which has been used to prepare dipeptidyl peptidase IV inhibitors derived from alagliptin.
Application
(R)-3-(Boc-Amino)piperidine is a chemical, is an organic synthesis intermediates and pharmaceutical intermediates, can be used in laboratory organic synthesis process and chemical pharmaceutical research and development process, as an intermediate in the production of alagliptin, ringliptin, to synthesize alagliptin benzoate.
References
[1] Toshiki M, Masaomi U, Sachiko S, et al. Preparation of 2-amino-5-phenylpyridine derivatives as inhibitors of IκB kinase β[P]. Jpn. Tokkyo Koho, 2004033455, 2004.
[2] D’Andrea G, Bussone G, Di Fiore P, et al. Pathogenesis of chronic cluster headache and bouts: role of tryptamine, arginine metabolism and α1-agonists[J]. Neurological Sciences, 2017, 38(1): 37-43.
[3] Yosuke W, Junichi Y, Tetsuya I. Preparation of piperidin-3-ylcarbamate compound and optical resolution method therefor[P]. PCT Int. Appl., 2009119700, 2009.
See also
Lastest Price from (R)-3-(Boc-Amino)piperidine manufacturers

US $1.00/g2025-08-11
- CAS:
- 309956-78-3
- Min. Order:
- 100g
- Purity:
- 99
- Supply Ability:
- 1000
US $0.00-0.00/kg2025-04-04
- CAS:
- 309956-78-3
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 1Ton