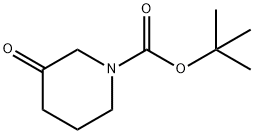
Synthesis and Application of N-Boc-3-piperidone
General description
N-Boc-3-piperidone is a very important intermediate of medicine, pesticide and other chemical additives. It can be used in laboratory research and development process and chemical and pharmaceutical synthesis process, mainly used for stereoscopic control synthesis of chiral compounds.
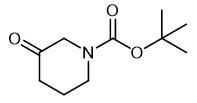
Fig. 1 The structure of N-Boc-3-piperidone.
Physicochemical property
N-Boc-3-piperidone is a clear colorless liquid with a melting point of 35-40℃ (lit.). Its density is about 1.099±0.06 g/cm3. The compound is insoluble in water. It is stored at 2-8°C
Synthetic routes
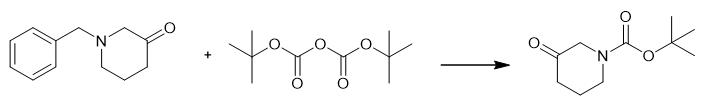
Fig. 2 The synthetic scheme 1 of N-Boc-3-piperidone.
To a solution of 1-benzyl-3-piperidone (102.6 g, 0.41 mol) in MeOH (1.4 L) was carefully added 10% palladium on carbon (11.6 g) under nitrogen, and the mixture was shaken under 55 psi of H2 overnight. The mixture was filtered through celite and the solvent concentrated in vacuo to give 72.1 g of a yellow-green solid that was used immediately. The product was dissolved in THF (2.5 L). Aq sat. NaHCO3 (3 L) and di-tert-butyldicarbonate (115.3 g, 0.53 mol) were added with THF (500 mL) and the mixture was stirred for 48 h. The mixture was partitioned between H2O (1 L) and EtOAc (2 L) and the layers separated. The aqueous layer was back-extracted with EtOAc (500 mL). The combined organics were washed with dilute HCl (0.5 N, 1 L) and brine (1 L), dried over MgSO4 and concentrated in vacuo. The residue was adsorbed onto silica gel (92 g) and purified by silica gel flash chromatography (1.9 kg), eluting by gradient of 25-40% EtOAc in hexane, to give 4. Clear oil. Yield 56.6 g, 99%. 1H NMR (300 MHz, CDCl3): δ = 4.04 (s, 2 H), 3.59 (t, J = 6.1 Hz, 2 H), 2.47 (t, J = 6.7 Hz, 2 H), 1.98 (m, 2 H), 1.47 (s, 9 H) [1].

Fig. 3 The synthetic scheme 2 of N-Boc-3-piperidone.
To a solution of 3-hydroxy-piperidine-1-carboxylic acid tert-butyl ester (Compound 3a) (0.25 g; 1.24 mmol) in anhydrous dichloromethane (9.0 mL) at 0° C. was added 1,1,1-tris(actyloxy)-1,1-dihydro-1,2-benzodioxol-3-(1H)-one (1.58 g; 3.72 mmol). The mixture was allowed to stir at room temperature under nitrogen for 2 h, to which was then added additional 1,1,1-tris(actyloxy)-1,1-dihydro-1,2-benziodoxol-3-(1H)-one (0.5 g; 1.18 mmol). Upon stirring for 20 h at room temperature, the reaction mixture was partitioned between dichloromethane and brine. The organic layer was washed with brine, dried over Na2SO4, filtered, and the solvent evaporated in vacuo to yield a crude oil. The crude oil was purified via flash column chromatography, eluting with 40% ethyl acetate in hexanes, to yield Compound 3b as an oil (0.24 g; 97% yield). 1H NMR (400 MHz, CDCl3): δ 4.0 (2H, br s), 3.60-3.57 (2H, m), 2.49-2.45 (2H, m), 2.01-1.95 (1H, m), 1.46 (9H, s) [2].
Application
1. Sitalide is the main inhibitor of dipeptidyl peptidase commercially available Sitagliptinintermediate, Trelagioptinsuccimate, Linagliptin, Alogliptiubenxoute, (R)-N-Boc-3-Aminopiperidine play a very important role in the synthesis of these dipeptidyl peptidase IV(DPP-IV) inhibitors. For example, in the Linagliptin technology roadmap published by Boehringer Ingelheim, Germany, (R)-N-Boc-3-Aminopiperidine is directly involved in the formation of the Linagliptin core skeleton. At present, the synthesis method of (R)-N-Boc-3-Aminopiperidine is mainly asymmetric synthesis. CN103588699 uses N-Boc-3-piperidone as raw material and reacts with (R)-α-methylbenzylamine as a chiral cogroup to obtain the intermediate enamine, and then hydrogenates the enamine through the control of the chiral cogroup. N-Boc-(R)-3- Aminopiperidine was obtained with the final removal of the chiral cogroup [3].
2. Piperazone and its derivatives are very important piperazone homologues, and many organic reactions can be induced by the activity of diphenyl in piperazone structure. Its derivatives have been found to have antibacterial, antitumor, alzheimer's disease and anesthesia activities, and are also one of the important drugs in the treatment of viral infections (including AIDI) and diabetes. For example, (S)-N-Boc-3-hydroxypiperidine can be used to synthesize an unnatural drug against congestive heart failure drug camorelin. (S)-N-Boc-3-hydroxypiperidine was prepared by asymmetric reduction of N-Boc-3-piperidone biocatalytically [4].
References
[1] Emmett G C, Cain G A, Estrella M J, et al. Efficient preparation of (3S)-3-(4-fluorobenzyl) piperidinium mandelate[J]. Synthesis, 2005, 2005(01): 92-96.
[2] Coats S, Bian H, Connolly P, et al. Pyrimidine compounds as delta opioid receptor modulators and their preparation and use in the treatment of opioid receptor mediated diseases [P]. PCT Int. Appl., 2011053705, 2011.
[3] Feng J, Tang Y, Xiong Y, Ai Z, She F. New aminotransferase polypeptide, for converting substrate N-Boc-3-piperidone into product (R)-1-Boc-3-amino piperidine [P]. Faming Zhuanli Shenqing, 111549011, 2020.
[4] Yan M, Zhang Z, Wei M, et al. Method for preparing (S)-N-Boc-3-hydroxypiperidine by enzymic asymmetric reduction[P]. Faming Zhuanli Shenqing, 105274160, 2016.
See also
Lastest Price from 1-Boc-3-piperidone manufacturers

US $1.50/g2025-06-24
- CAS:
- 98977-36-7
- Min. Order:
- 1g
- Purity:
- 99.0% Min
- Supply Ability:
- 10 Tons

US $0.00-0.00/KG2025-01-03
- CAS:
- 98977-36-7
- Min. Order:
- 1g
- Purity:
- 99%
- Supply Ability:
- 2000tons