Synthesis and Application of Lenvatinib Mesylate
General description
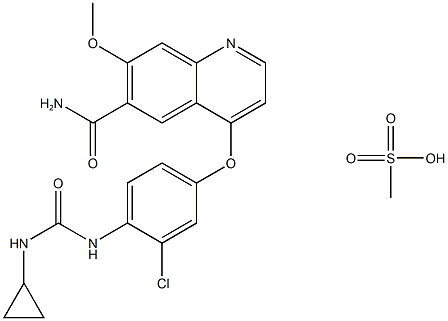
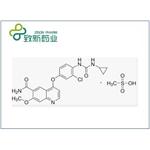
Lenvatinib mesylate is the mesylate of lenvatinib, a multi-target tyrosine kinase inhibitor developed by Eisai, Japan, which has a novel binding mode, In addition to inhibiting other tyrosine kinases associated with chemicalbook angiogenesis and oncogenic signaling pathways involved in tumor proliferation, it can also selectively inhibit the kinase activity of vascular endothelial growth factor (VEGF) receptors, such as VEGF-1 VEGF-2 and VEGF- 3.
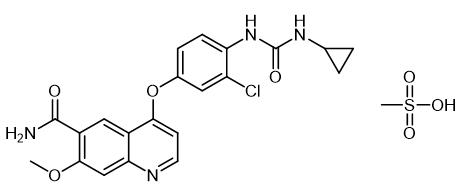
Fig. 1 The structure of Lenvatinib Mesylate.
Physicochemical property
Lenvatinib Mesylate is a white solid with a melting point greater than 220°C. It is slightly soluble in DMSO and methanol.
Synthetic routes
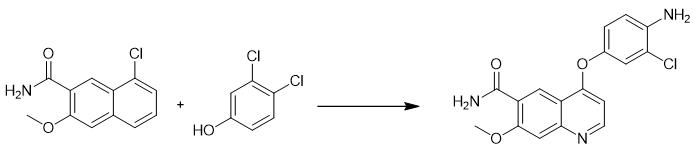
Fig. 2 The synthetic step 1 of Lenvatinib Mesylate.
Under nitrogen protection, add 200 mL dimethyl sulfoxide to a 500 mL three-mouth bottle, start stirring, then add 20.00 g 4-chloro-7-methoxy quinoline-6-carboxyamide, 18.20 g 4-amino-3-chlorophenol and 14.22 g potassium tert-butanol successively. After feeding, the temperature was raised to 65°C and the mixture was kept warm and stirred for 19 hours. The reaction system was poured into pure water, and a large amount of solid precipitate was filtered. The filter cake was washed with pure water, and the brown solid 25.07 g 4-(4-amino-3-chlorophenoxy) -7-methoxy quinoline-6-carboxyamide was obtained by air drying for 1 ~ 2 hours at 60 °C. Yield: 86.32% [1].
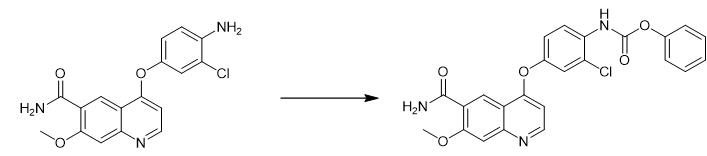
Fig. 3 The synthetic step 2 of Lenvatinib Mesylate.
In the 500 mL three-mouth flask, add 250 mL N-methylpyrrolidone, turn on stirring, then add 11.50 g pyridine and 25.00 g 4-(4-amino-3-chlorophenoxy)-7-methoxyquinoline-6 successively - Carboxylic acid amide. Under the ice-water bath, 4.94 g of phenyl chloroformate was added dropwise to the system, and after the dropwise addition, the system was warmed to room temperature. In the reaction system, drip pure water, drop time 1-2 hours, filter, rinse, under 60 °C of conditions, blast drying 2 hours, obtain 33.74 g (4-((6-carboxylic acid carboxamido- 7-Methoxyquinolin-4-yl)oxy)-2-chlorophenyl)phenylcarbamate brown powder. Yield: 81.26% [1].
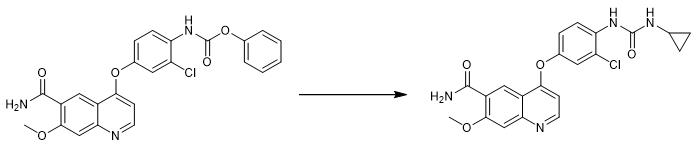
Fig. 4 The synthetic step 3 of Lenvatinib Mesylate.
Add 300 mL N-methylpyrrolidone to a 1000 mL three-mouth flask, start stirring, and add 30.00 g(4-((6-carboxylate formamyl-7-methoxyquinoline-4-yl) oxygen) -2-chlorophenyl) paraben. At room temperature, 4.43 g cyclopropyl amine was added to the body system for 1 hour. After dripping, continue to keep warm and stir overnight. 300 mL pure water was added to the reaction system and stirred until a large amount of solid was precipitated (about 1-2 hours). The mixture was stirred for another hour, filtered and washed, and then air-dried at 60 °C for 12 hours to obtain 27.50 g of crude product. 21.08 g of white solid 4-(3-chlorine 4-(N '-cyclopropyl urea) phenoxy) -7-methoxyquinoline-6-carboxyamide (LVTN-3) was obtained by recrystallization of ethanol with a yield of 76.35% [1].
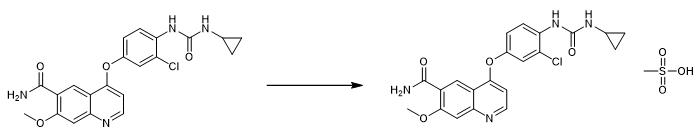
Fig. 5 The synthetic step 4 of Lenvatinib Mesylate.
Under the protection of nitrogen, 100 mL acetic acid and 2.70 g mesylate were added successively to 250 mL three-mouth bottle, and then 10.0 g LVTN-3 was added to the system under the condition of 40 °C temperature control. After stirring for 1-2 hours, the mother liquor was collected by filtration. The mother liquor was transferred to 500 mL three-mouth bottle under the protection of nitrogen, and the temperature control was 40 °C. Add 170 mL of n-propanol to the system by drop, and control the drop time for 1-2 hours. After filtration, the cake was washed with 70 mL N-propanol to obtain the acetic acid compound of levatinib mesylate. Under the protection of nitrogen, 100 mL ethanol was added into a 250 mL three-mouth flask, and the mixture was started to stir. Under the condition of temperature control of 40°C, levatinib mesylate acetic acid compound was added. After stirring for 36 hours, the filter cake was washed with ethanol, and the finished product of levatinib mesylate was obtained by air drying at 60°C for 12 hours. It is white powder with yield of 83.49% [1].
Application
Crystal Structure, Equilibrium Solubility, and Stability Study
Lenvatinib is a cancer treatment drug approved by the U.S. Food and Drug Administration, which is a multitarget tyrosinase inhibitor. The commercially available form is its mesylate. In this work, five lenvatinib mesylate (LEM) solid-state forms, including two polymorphs (LEM-A, LEM-C) and three pseudopolymorphs (a hydrate (LEM-H) and two iso-structural solvates (LEM-H-EA and LEM-H-THF)), were prepared and characterized using various methods. Powder diffractions of LEMA and LEM-C have been reported, but their crystal structures were reported for the first time by us. Hydrate and two iso-structural solvates were also first prepared and characterized by thermal analysis, X-ray diffraction, and so forth. The mutual transformation of different forms was studied. Two solvates easily lost their solvent and turned into LEM-B, which provided the possibility for the preparation of LEM-B. In addition, thermal stability and solubility of LEM-A, LEM-C, and LEM-H were determined, along with the respective molecular conformation, their inter- and intramolecular interactions, and packing arrangements [2].
Anticancer
Hepatocellular carcinoma (HCC) is a worldwide healthcare problem, with a rising incidence. In its advanced stage, the prognosis of untreated HCC is very poor. Only in 2007, after a long series of failed trials, the multi-tyrosine kinase inhibitor sorafenib demonstrated its superiority over placebo, becoming the first approved frontline therapy for advanced HCC. For a decade, all of the frontline trials using sorafenib as a comparator systematically failed, leaving this drug as the only available treatment in this setting. In 2018, lenvatinib mesylate (another multitarget tyrosine kinase inhibitor) demonstrated noninferiority compared to sorafenib in the phase III, randomized, controlled REFLECT trial. Currently, lenvatinib represents the only available alternative to sorafenib as a frontline systemic treatment of advanced HCC. In this monograph, we review the main preclinical and clinical evidence that emerged in the trials of lenvatinib, with particular attention to the features differentiating this drug from sorafenib [3].
The gelation of lenvatinib mesylate
Lenvatinib mesylate (LM) is a first-line anticancer agent for the treatment of unresectable hepatocellular carcinoma, while it formed viscoelastic hydrogel when contacting with aqueous medium, which would significantly hinder its in vitro dissolution. The aim of this study was to systematicly explore the gelation mechanism and gel properties via thermal analysis, rheology, morphology and spectroscopy studies. The formed hydrogel was found to be composed of a new polymo
Related articles And Qustion
See also
Lastest Price from lenvatinib Mesylate manufacturers

US $0.00-0.00/KG2025-04-21
- CAS:
- 857890-39-2
- Min. Order:
- 1g
- Purity:
- 99%min
- Supply Ability:
- 10 KGS
US $0.00-0.00/kg2025-04-21
- CAS:
- 857890-39-2
- Min. Order:
- 1kg
- Purity:
- >99% by HPLC
- Supply Ability:
- 10kg/month